Previously, the world of material sciences had firmly established that any material that conducts electricity must also be a good conductor of heat, as dictated by the Wiedemann-Franz Law. But the scientists at the Lawrence Berkeley National Laboratory (Berkeley Lab) and University of California, Berkeley, USA, have recently discovered some properties of a material which shook these basics to the core. They created a material called vanadium dioxide that can conduct electricity without conducting heat!
The findings can be applied to a host of applications such as thermoelectric systems which can convert waste heat from engines and machines into electricity. Metallic vanadium dioxide is already famous for its ability to switch from an insulator to metal when heated to the temperature of 67 degrees Celsius. It has a unique electronic configuration which makes this feat plausible.
Junqiao Wu, a physicist at Berkeley Lab, said,
“It shows a drastic breakdown of a textbook law that has been known to be robust for conventional conductors. The discovery is of fundamental importance to understand the basic electronic behaviour of novel conductors.”
The researchers used simulations and X-ray scattering experiments to isolate the vibration of the material’s crystal lattice, called phonons, from the movement of electrons. This isolation shows that the material could transport charge without heating up.

The researchers also realized that the thermal conductivity attributed to these electrons is already ten times smaller than what the Wiedemann-Franz Law dictates.
“For electrons, heat is a random motion. Normal metals transport heat efficiently because there are so many different possible microscopic configurations that the individual electrons can jump between,” said Wu.
“In contrast, the coordinated, marching-band-like motion of electrons in vanadium dioxide is detrimental to heat transfer as there are fewer configurations available for the electrons to hop randomly between,” he said.
The mystery doesn’t end here. The amount of electric and heat conductivity of other materials can also be tuned by mixing vanadium dioxide with them in selected proportions. The researchers lowered the phase transition temperature of the material by doping a single crystal of vanadium dioxide with the metal tungsten. By doing so, the electrons in the metallic phase get better at conducting heat.
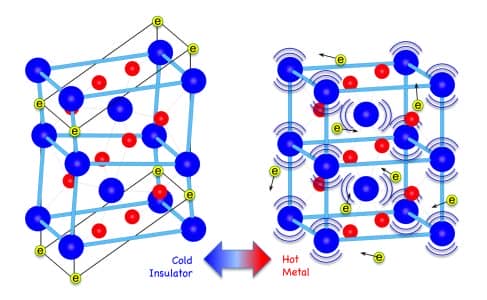
This technique allowed the researchers to manage the amount of heat dissipated by vanadium dioxide by playing with its phase from an insulator to metal. The researchers mentioned that the metallic Vanadium dioxide could be used in a host of applications, such as scavenging or dissipating heat in engines and developing window coatings that improve the energy efficiency in buildings.
The findings were published in the journal Science.
What are your thoughts on this incredible discovery? Comment below!