Engineers at Drexel University have made a breakthrough that will take the Lithium-sulfur batteries closer to commercial use, by leveraging a rare chemical phase of sulfur to prevent damaging chemical reactions.
These batteries are more environment-friendly and provide massive performance gains. They have the potential to store several times the energy of today’s lithium-ion batteries. However, the formation of chemical compounds called polysulfides is creating problems for them.
As the battery operates, these make their way into the electrolyte – the solution that carries the charge back and forth between the anode and cathode – where they trigger chemical reactions that compromise the battery’s capacity and lifespan. Scientists have had some success swapping out the carbonate electrolyte for an ether electrolyte, which doesn’t react with the polysulfides. But this leads to other issues as the ether electrolyte itself is highly volatile and has components with low boiling points, which means that the battery could quickly fail or meltdown if warmed above room temperature.
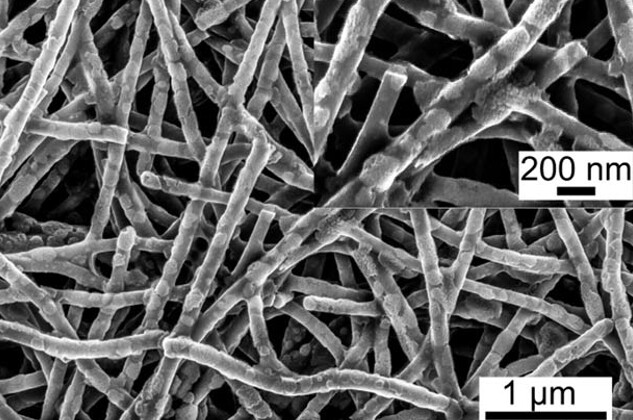
To combat this issue, engineers are designing a new cathode, which can work with the carbonate electrolytes already in commercial use. This cathode is made from carbon nanofibers and had already been shown to slow the movement of polysulfides in an ether electrolyte.
“Having a cathode that works with the carbonate electrolyte that they’re already using is the path of least resistance for commercial manufacturers,” said lead researcher Vibha Kalra. “So rather than pushing for the industry adoption of a new electrolyte, our goal was to make a cathode that could work in the pre-existing Li-ion electrolyte system.”
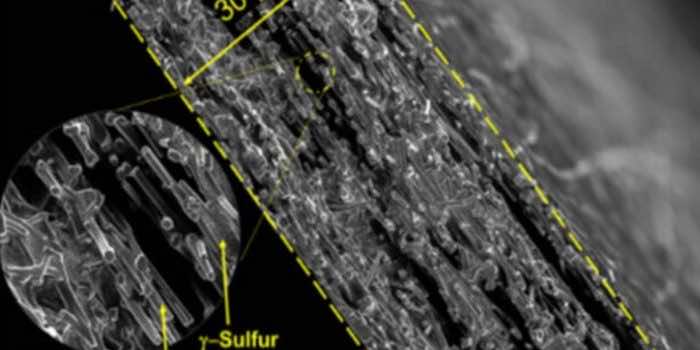
The scientists attempted to confine the sulfur in the carbon nanofiber mesh to prevent dangerous chemical reactions using a technique called vapor disposition. It turned it into something called monoclinic gamma-phase sulfur, a slightly altered form of the element. This chemical phase of sulfur had only been produced at high temperatures in the lab or observed in oil wells in nature.
“At first, it was hard to believe that this is what we were detecting because in all previous research monoclinic sulfur has been unstable under 95 °C (203 °F),” said Rahul Pai, co-author of the research. “In the last century there have only been a handful of studies that produced monoclinic gamma sulfur and it has only been stable for 20-30 minutes at most. But we had created it in a cathode that was undergoing thousands of charge-discharge cycles without diminished performance – and a year later, our examination of it shows that the chemical phase has remained the same.”
The cathode remained stable across a year of testing and 4,000 charge-discharge cycles, which the scientists say is equivalent to 10 years of regular use.
“While we are still working to understand the exact mechanism behind the creation of this stable monoclinic sulfur at room temperature, this remains an exciting discovery and one that could open a number of doors for developing more sustainable and affordable battery technology,” Kalra said.
The research was published in the journal Communications Chemistry.