Scientists have just created the thinnest film of liquid ever in the lab environment. The new research has gained a lot of popularity, and the researchers explain a simpler way to understand what they have accomplished. When rain falls on someone’s iPhone; a small stream of beads form on the screen of the phone which can be shaken off and will not cause any damage to the phone. On the other hand, if someone takes their phone with them in the shower, it will be covered with a thin layer of water due to stream. The researchers said that these two concepts contribute to the same idea which they have introduced.
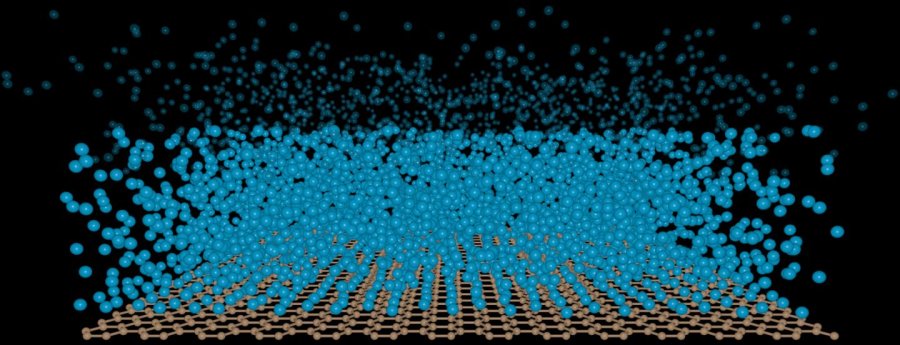
Adrian Del Maestro, the co-author of the study, said, “These are two extreme examples of the physics of wetting. If interactions inside the liquid are stronger than those between the liquid and surface, the liquid atoms stick together, forming separate droplets. In the opposite case, the strong pull of the surface causes the liquid to spread, forming a thin film.” Physicists theorized that there could be a third possibility of wetting. They developed the idea of ‘critical wetting.’ It happens when atoms of liquid will form on a film’s surface but will stop after it gets a few atoms thick.
Soviet scientist, Evgeny Lifshitz, understood the concept, but like many other scientists, he was also confused if the concept was real or not. If critical wetting was real, it could be easily tested in a laboratory setting. The 2010 Nobel Prize award went to two Russian scientists for the creation of graphene. The Vermont research team used this material to prove that ‘critical wetting’ was real. In the last few years, graphene has proven to be a super material. It conducts the electricity well, interacts with water in unique ways, and can be tailored to do about anything which scientists across a variety of fields can need.
For Del Maestro and his team, graphene was the ideal surface to test for critical wetting. The physicists compared various amounts of hydrogen, helium, and nitrogen behavior upon exposure to graphene. They also used vacuum in other conditions. The team discovered a liquid layer of the various gases which would form on the ultra-thin graphene. A team member Valeri Kotov pointed out that the film stops growing when it is ten or twenty atoms thick. The film sticks to graphene thanks to Van Der Waals forces. The weak forces of the liquid to the graphene are not impressive when they are compared to the strength with which water clings to objects. Getting the weak forces to stick in a laboratory setting was a challenge too. Sengupta said, “What’s important is that we can tune this thickness.” The discovery will not reshape water-proof materials or water-proof electronics; the team said that it would give other engineers the ability to understand and control critical wetting. It also provides significant steps forward in what researchers know about graphene.