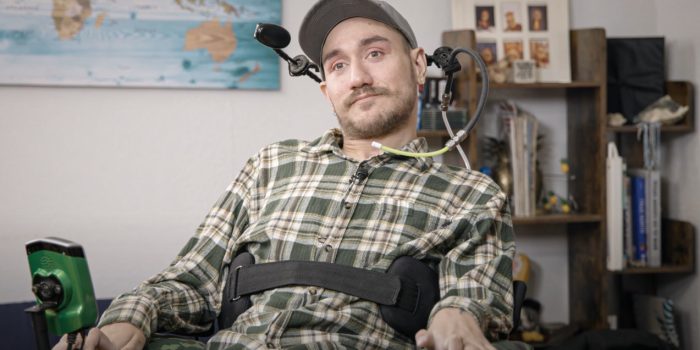
Noland Arbaugh experienced a life-altering swimming accident in 2016, which left him a quadriplegic. In January 2024, he became the first person to receive Elon Musk’s Neuralink brain implant, the Link, designed to grant telekinetic abilities. This implant has profoundly transformed Arbaugh’s life, providing him with new purpose and significantly enhancing his quality of life.
“I didn’t have anything to wake up for in the morning, and this has changed that for me,” Arbaugh said in a Good Morning America interview.
The Link implant is a small device, about the size of a large coin, featuring 1,024 electrodes on 64 leads or “threads,” which connect to brain regions controlling motor functions. These threads, thinner than a human hair, require highly precise placement, a task performed by Neuralink’s R1 robot. The robot’s needle tips are nearly the size of a red blood cell, minimizing brain tissue damage during implantation.

Arbaugh’s surgery was successful, allowing him to leave the hospital the next day without any adverse effects. Within a month, he could control his computer mouse via Bluetooth using only his thoughts. The implant, located just beneath his scalp, is nearly invisible and uses induction charging, with a battery life of approximately eight hours. Arbaugh enjoys playing video games like Chess and Civilization VI using his mind, though he occasionally drains the battery during extended gaming sessions.
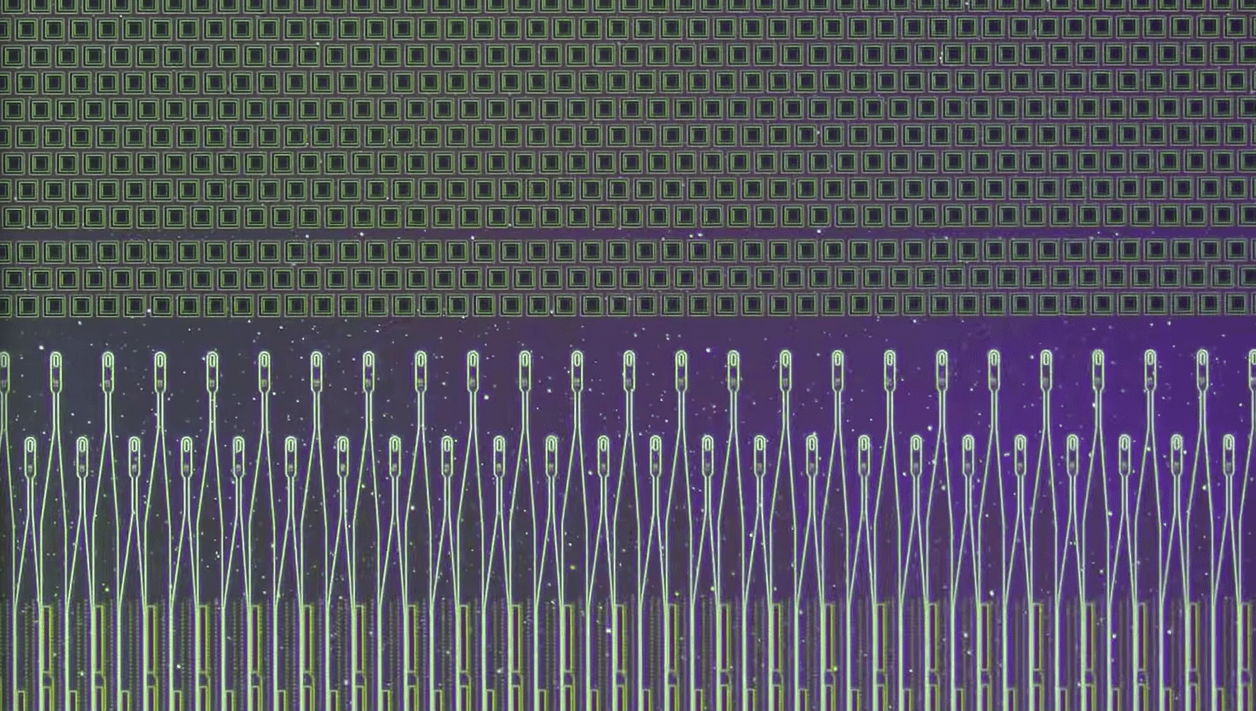
Recently, Arbaugh experienced issues as some implant threads retracted from his brain tissue, reducing the number of functional electrodes and slowing his control over the computer. Neuralink addressed this by enhancing the implant’s algorithm, improving sensitivity to neural signals, and refining the user interface. These adjustments not only restored but surpassed Arbaugh’s initial performance.
“In the weeks following the surgery, a number of threads retracted from the brain, resulting in a net decrease in the number of effective electrodes,” Neuralink said in a blog post. “This led to a reduction in BPS. In response to this change, we modified the recording algorithm to be more sensitive to neural population signals, improved the techniques to translate these signals into cursor movements, and enhanced the user interface. These refinements produced a rapid and sustained improvement in BPS, that has now superseded Noland’s initial performance.”

The thread retractions were likely caused by pneumocephalus, a condition where air is trapped in the skull post-surgery. Neuralink had considered removing the implant but managed to resolve the data-loss issues through algorithm modifications. Despite the initial setback, Arbaugh expressed relief and gratitude, noting the implant’s role in reconnecting him with his world and providing independence.
Facing the the initial news that his implant may be removed, Arbaugh was very emotional saying, “it was very, very hard to give up all of the amazing things that I was able to do. I think I had cried afterwards.” He went on to say “[The Link] has helped me reconnect with the world, my friends, and my family. It’s given me the ability to do things on my own again without needing my family at all hours of the day and night.”

Currently, Arbaugh is doing well, with no health concerns despite some displaced threads. Neuralink, led by Musk, is seeking a second human trial participant, focusing on individuals with quadriplegia or ALS. Future iterations of the Link aim to restore movement electronically in people with spinal cord injuries, pushing beyond mere cursor control.
However, Neuralink has faced controversy, particularly regarding the reported deaths of over a dozen monkeys during early trials. Musk insists that only terminally ill monkeys were used, though conflicting reports from Neuralink employees have surfaced. Despite these issues, the FDA has approved human testing, suggesting that this technology could mark a significant advancement for individuals with severe disabilities.