A Dye-sensitized solar cell has been created by the researchers at Ohio State University, having the ability to store its own power by “breathing” air in its surrounding to decompose and re-form lithium peroxide. The creators of the device, which is actually a combination of a battery and a solar cell, plan to reduce renewable energy costs by 25 percent.
“The state of the art is to use a solar panel to capture the light, and then use a cheap battery to store the energy,” OSU professor Yiying Wu explained. “We’ve integrated both functions into one device. Any time you can do that, you reduce cost.”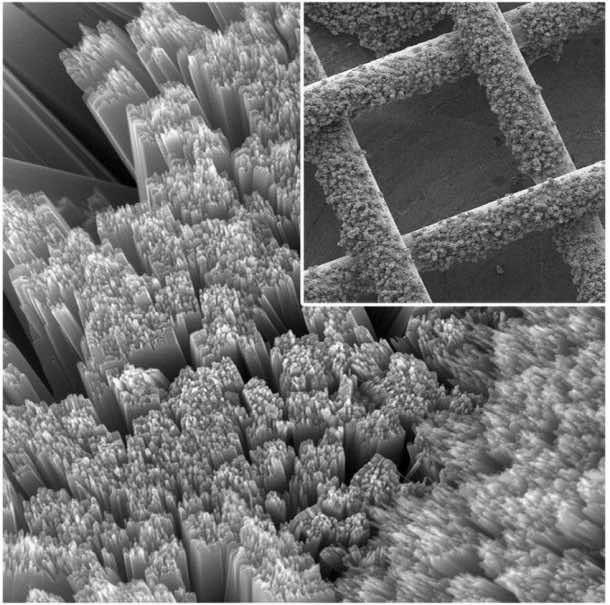
The device is designed to use three electrodes, instead of the four that a battery usually holds. At the bottom of these a lithium plate is laid, upon which there is a layer of electrolyte, a thin sheet of porous carbon and then another layer of electrolyte. At the top of all this, a permeable titanium gauze mesh contains a dye-sensitive titanium dioxide photo electrode resembling blades of grass at the 1um scale in look and produces tri-iodide ions under light. These ions are then spread to the oxygen electrode surface via an iodide, “shuttle”, where they are oxidized into lithium peroxide.
Lithium peroxide is then chemically decomposed into lithium ions and oxygen using the electrons of the connected battery, the oxygen is released into air and lithium ions are stored as lithium metal. When the discharging starts, oxygen is collected from the environment and is consumed to form lithium peroxide – and the cycle repeats.
A red dye named ruthenium compound is used to coat the mesh, tuning the wavelength of the light entering. The dye absorbs light and electrons are released by it, which produces an electric current as more electrons are pulled from the iodide solution, replacing them. The drawback is the short lifetime of this rechargeable solar cell, as the dye runs out in just eight hours of charge and discharge cycles.
The initial tests have been done by the researchers using hematite (rust) photo electrode instead of the dye-sensitized titanium oxide, which seems to be offering comparable efficiency and “remarkably better” stability that may enable the battery to keep its lifetime in the same range as the rechargeable batteries in the market do.
If they can settle on a material that gets similar efficiency (about 100 percent of electrons retained, as compared to the 80 percent retaining in typical cells) to this titanium oxide version, while providing the lifetime of a number of years, this invention of OSU researchers can have huge positive impacts in the field of renewable energy. Usual acceptance of renewables is currently confined to energy generation and most of the cost comes from the energy stored through the grid that loses more than 25 percent of the electricity generated.
Further research is being funded by US Department of Energy, while OSU will license the solar battery to industry and will pursue to improve it performance-wise. A pretty cool new invention, isn’t it?


