Researchers from the University of Birmingham have designed a novel adaptation for existing iron and steel furnaces that could reduce carbon dioxide (CO2) emissions from the steelmaking industry by nearly 94%.
This decarbonization idea is something very rare but looks good for the environment as well as business. Not only does this promises to reduce carbon emissions but also produces oxygen as a byproduct. This method uses the existing blast furnaces without replacing them completely.

Iron and steel production plants are responsible for somewhere around 8% of global carbon emissions, making them the single biggest source of industrial greenhouse gases. To reduce these carbon emissions, research has been searching for a solution for decades. The path to a 100% clean future is clear enough; ditch the blast furnaces and baked-coal coke reductants, and replace them with electric arc furnaces running on clean energy, and green hydrogen reductants.
But with enormous amounts of steel being produced every year globally i.e., around two billion tons, switching to an electric arc furnace will cost between US$1.1 and 1.7 billion. Hence, we won’t be replacing blast furnaces anytime soon.
What this new retrofit system from the University of Birmingham replaces about 90% of the coke used in the blast furnace with direct carbon monoxide injection.
The novel recycling system captures the CO2 from the top gas and reduces it to CO using a crystalline mineral lattice known as a ‘perovskite’ material. The material was chosen as the reactions take place within a range of temperatures (700-800°C) that can be powered by renewable energy sources and/or generated using heat exchangers connected to the blast furnaces.
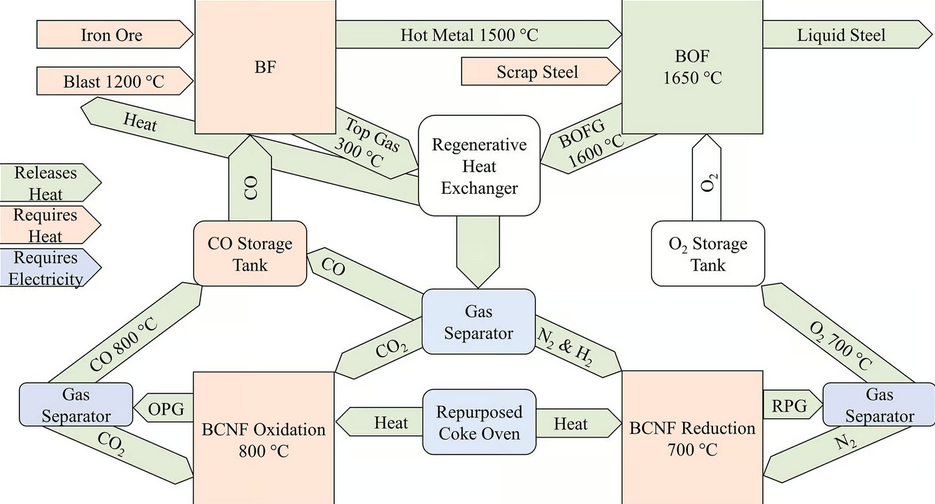
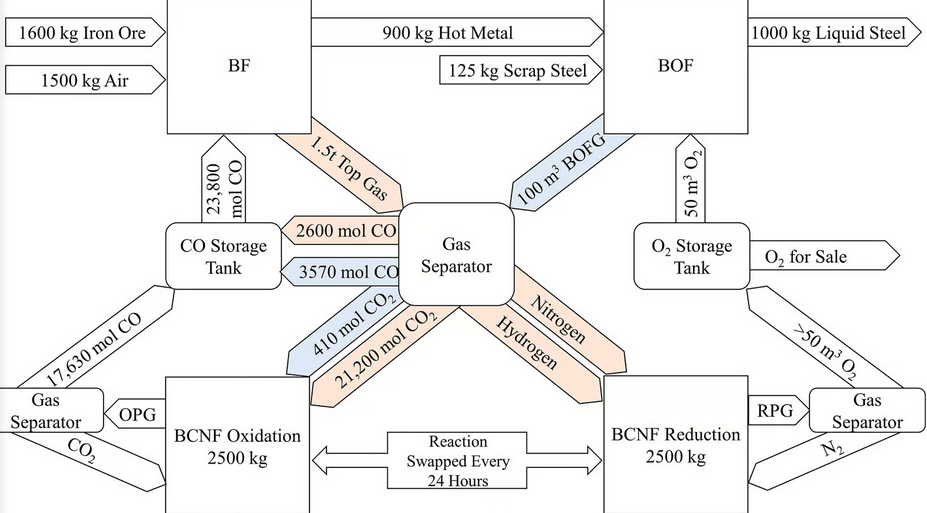
Under a high concentration of CO2, the perovskite splits CO2 into oxygen, which is absorbed into the lattice, and CO, which is fed back into the blast furnace. The perovskite can be regenerated to its original form in a chemical reaction that takes place in a low-oxygen environment. The oxygen produced can be used in the basic oxygen furnace to produce steel.
This system devised by the University of Birmingham shows that if implemented in the UK alone, it could deliver cost savings of £1.28 billion in 5 years while reducing overall UK emissions by 2.9%.
Iron and steelmaking is the biggest emitter of CO2 of all foundation industrial sectors, accounting for 9% of global emissions. According to the International Renewable Energy Agency (IRENA), it must achieve a 90% reduction in emissions by 2050 to limit global warming to 1.5°C.


