Researchers from Stanford University have come up with a type of non-flammable electrolyte for lithium-ion batteries. The new polymer-based Solvent-Anchored non-Flammable Electrolyte, abbreviated as SAFE, enables electronic devices to work at high temperatures without igniting.
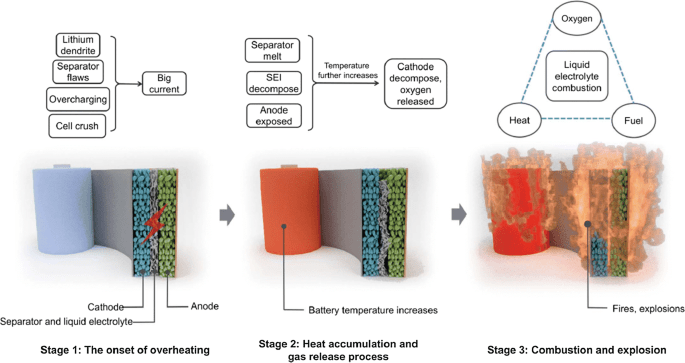
Lithium-ion batteries have a risk of starting a fire as they can catch fire at high temperatures. This is because the electrode used inside them is flammable.
“One of the biggest challenges in the battery industry is this safety issue, so there’s a lot of effort going into trying to make a battery electrolyte that is safe,” said Rachel Z Huang, a graduate student at Stanford University and first author of the new study.
Traditional lithium-ion battery electrolytes are made of lithium salt dissolved in a liquid organic solvent. At temperatures above 140 degrees Fahrenheit (60 degrees Celsius), the solvent starts to evaporate, transforming from liquid to gas, and causing the battery to swell up. The gas then catches fire and the battery explodes.
The team led by Yi Cui, a professor at SLAC National Accelerator Laboratory and Stanford, went to produce a polymer-based electrolyte that could offer both safety and performance.
He did this by adding a particular lithium salt (LiFSI) to a polymer-based electrolyte, bumping the mix from less than half of the electrolyte’s weight to 63 percent. The additional salt stops the electrolyte from evaporating in high temperatures and stops them from catching fire. In temperature tests, the lithium-ion battery continued functioning at temperatures as high as 212 degrees Fahrenheit (100 degrees Celsius).
“I just wanted to see how much I could add and test the limit,” Huang said.

One important thing to note is that this new electrolyte has the same consistency as the traditional ones. This will make it easy to integrate into conventional structures, unlike other experimental, non-flammable electrolytes.
This could also be used in electric cars.
“This very exciting new battery electrolyte is compatible with the existing lithium ion-battery cell technology and would make big impacts on consumer electronics and electrical transportation,” said Professor Yi Cui.
The study was published in the journal Matter.


