Silicon, the standard semiconducting material used in a host of applications—computer central processing units (CPUs), semiconductor chips, detectors, and solar cells—is an abundant, naturally occurring material. However, it is expensive to mine and purify.
Perovskites—a family of materials nicknamed for their crystalline structure—have shown extraordinary promise in recent years as a far less expensive, equally efficient replacement for silicon in solar cells and detectors. Now, a study led by Chunlei Guo, a professor of optics at the University of Rochester, suggests perovskites may become far more efficient.
Perovskites are one of the most promising new materials for solar cell technology and now a new way to more than triple the material’s efficiency by adding a layer of reflective silver underneath it has been developed.
“No one else has come to this observation in perovskites,” Guo says. “All of a sudden, we can put a metal platform under a perovskite, utterly changing the interaction of the electrons within the perovskite. Thus, we use a physical method to engineer that interaction.”
Perovskites and other photovoltaic materials generate electricity by allowing sunlight to excite electrons in the material, causing them to jump out of their atoms, ready to be guided to generate an electrical current. But sometimes, electrons fall back into the “holes” they left behind, reducing the overall current and as such the efficiency of the material. This is what’s known as electron recombination.
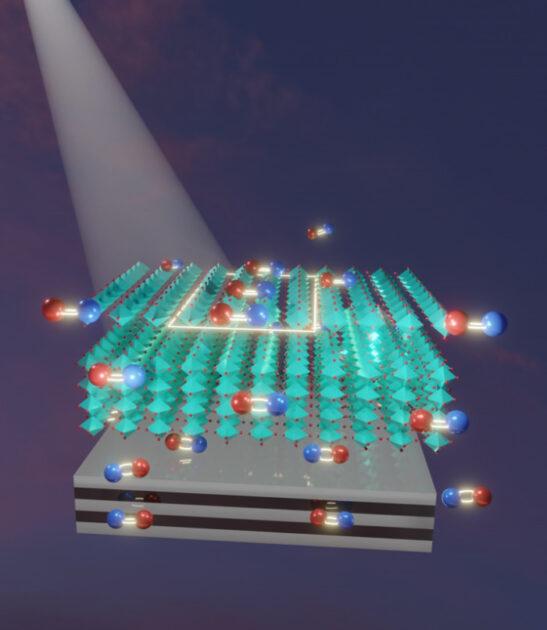
In a solar cell, photons from sunlight need to interact with and excite electrons, causing the electrons to leave their atomic cores and generate an electrical current, Guo explains. Ideally, the solar cell would use materials that are weak to pull the excited electrons back to the atomic cores and stop the electrical current.
Guo’s lab demonstrated that such recombination could be substantially prevented by combining a perovskite material with either a layer of metal or a metamaterial substrate consisting of alternating layers of silver, a noble metal, and aluminum oxide, a dielectric.
The result was a significant reduction of electron recombination through “a lot of surprising physics,” Guo says. In effect, the metal layer serves as a mirror, which creates reversed images of electron-hole pairs, weakening the ability of the electrons to recombine with the holes.
The lab was able to use a simple detector to observe the resulting 250 percent increase in the efficiency of light conversion.
“As new perovskites emerge, we can then use our physics-based method to further enhance their performance,” Guo says.


