In a recent study in Nature Sustainability, researchers introduce a groundbreaking design for a self-extinguishing rechargeable battery, heralding a significant leap in safety measures against fires and explosions linked to overheating.
The study showcases the successful replacement of the commonly used highly combustible electrolyte in rechargeable batteries with materials reminiscent of those in commercial fire extinguishers. This new electrolyte, derived from modifying affordable commercial coolants, exhibits nonflammable characteristics, heat resistance, and compatibility with diverse battery chemistries, thus enabling the battery to self-extinguish internal fires effectively across a wide temperature range.
Crucially, safety validation tests conducted in laboratory conditions demonstrated the exceptional performance of batteries equipped with this novel electrolyte, showcasing proficient heat dissipation capabilities and resilience to internal short circuits. Notably, the batteries withstood the rigorous nail penetration test, a standard assessment method for lithium-ion battery safety, without catching fire, highlighting their robustness in hazardous scenarios.
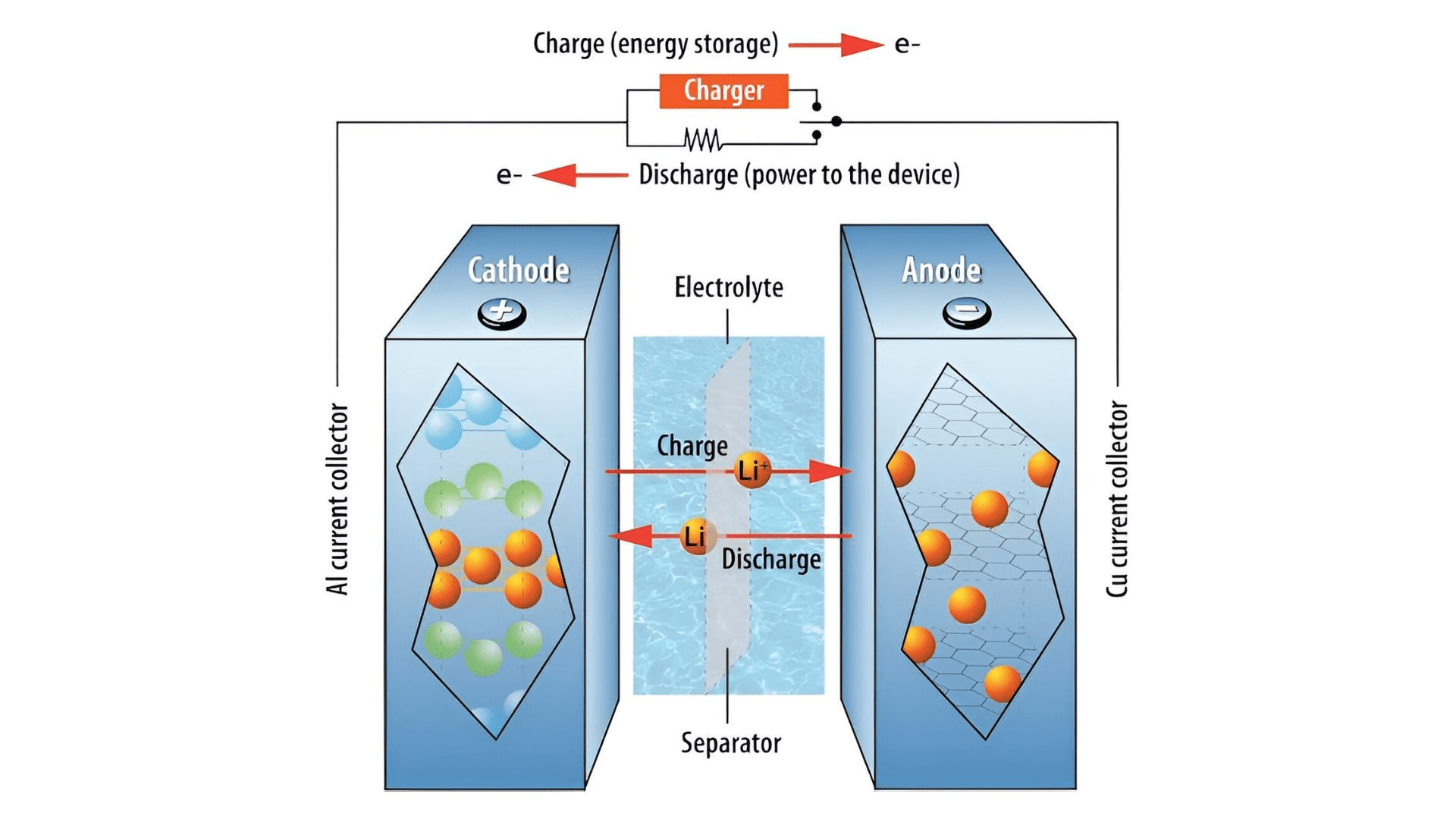
Integrating this innovative battery design addresses critical safety concerns associated with lithium-ion batteries, which are prevalent in various electronic devices and electric vehicles. These batteries are susceptible to temperature fluctuations during charging and discharging cycles, posing risks to safety and longevity. Moreover, the risk of dendrite formation during rapid charging exacerbates safety concerns.
By prioritizing the development of a nonflammable electrolyte capable of dissipating heat effectively and enduring extreme temperature differentials, the researchers aim to enhance battery safety while addressing environmental sustainability concerns. However, challenges related to the environmental impact and cost of conventional nonflammable organic solvents persist, prompting ongoing exploration of sustainable alternatives.
The study underscores the importance of investigating alternative alkali metal ions, such as potassium or sodium, to mitigate the scarcity of lithium in the Earth’s crust. Although the focus primarily revolves around self-extinguishing potassium-ion batteries, the electrolyte also exhibits compatibility with lithium-ion batteries. Future research aims to assess its applicability to emerging battery types, including sodium-ion, aluminium-ion, and zinc-ion batteries, with the overarching goal of developing practical, environmentally friendly, and sustainable battery solutions.
The adaptability of this alternative electrolyte to existing battery production lines offers a promising pathway for seamless integration into current lithium-ion battery facilities, potentially revolutionizing battery safety across various industries.


