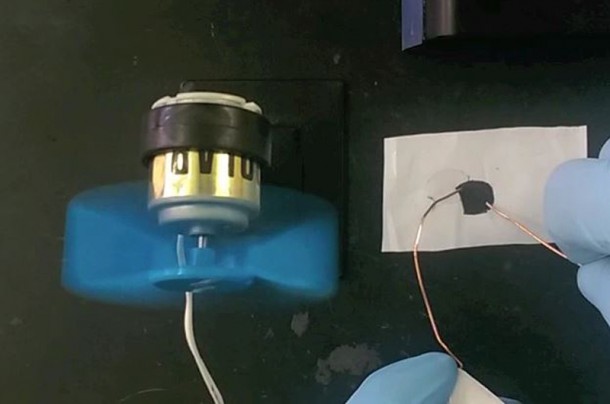
A team of researchers at Drexel University has come up with a conductive clay that qualifies as an “exceptionally viable candidate” for replacement of the current electrode materials that are being used in the batteries and super-capacitors. The material is called MXene and pronounced as “mex-een”.

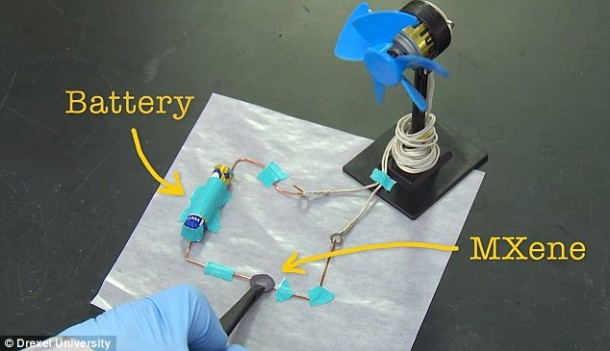
The three properties that set the invention apart are listed below:
- The material is hydrophilic (loves water) unlike graphene.
- Being hydrophilic allows it to be highly ductile.
- The material has an amazing capacitance of 900 F/cm3 and that too, at the first attempt by the team.


According to the researchers, the material has not lost any capacitance even after 10,000 charge cycles. MXene was discovered back in 2011 first and is essentially made up of 2D titanium carbide that has been derived from MAX phases. This particular material is new and unique because the team has managed to make it as a clay. Being manufactured as clay renders it far more useful while also making it safer and quicker as compared to the previous means of creating MXene. Moreover, this method makes use of readily available materials. It took researchers a time span of 1 day to create a MXene electrode before, and now the process can be completed in 15 minutes.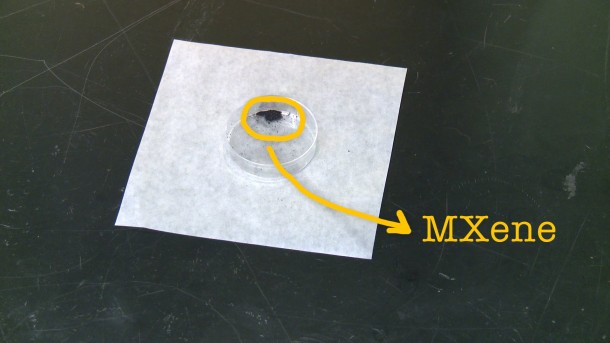
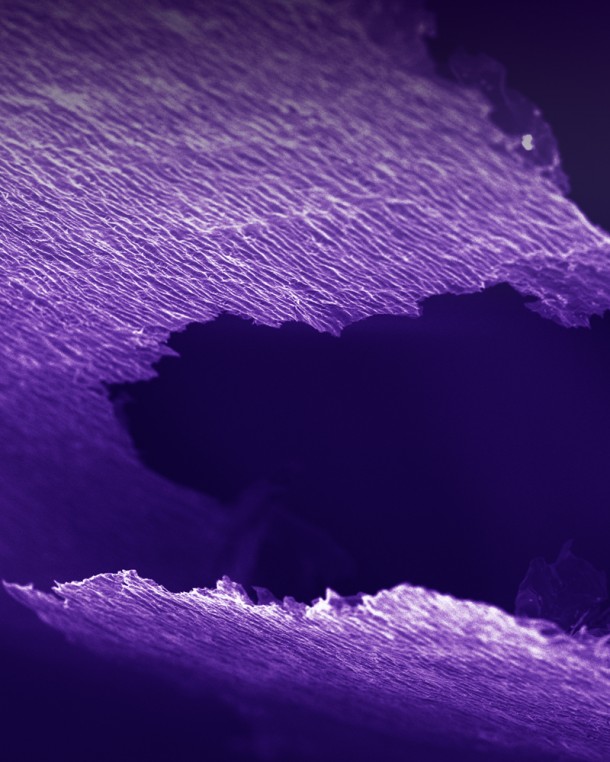
As of now, the research is in early stages, hence it is hard to say when these electrodes shall find their way into the market. Nonetheless the researchers are really excited and eager about it. Doctoral student Maria Lukatskaya said, “We’re talking about quite a special lump of clay here.” Let’s see when this special lump of clay makes it to the real world and how well it performs.