In the ongoing battle against Parkinson’s disease, a California biotech firm has illuminated a path toward a potential breakthrough. Through a groundbreaking experiment involving lab-made neurons, this company has taken a significant step forward in treating this debilitating illness.
The trial, orchestrated by BlueRock Therapeutics, a subsidiary of pharmaceutical giant Bayer, enlisted 12 individuals grappling with Parkinson’s—a condition characterized by dopamine deficiency. The study, initially designed to assess safety, has yielded promising results.
After a year of implanting these artificially created neurons, not only have they demonstrated resilience within the patients’ brains, but there are also early indications that they might be mitigating the symptoms of Parkinson’s.
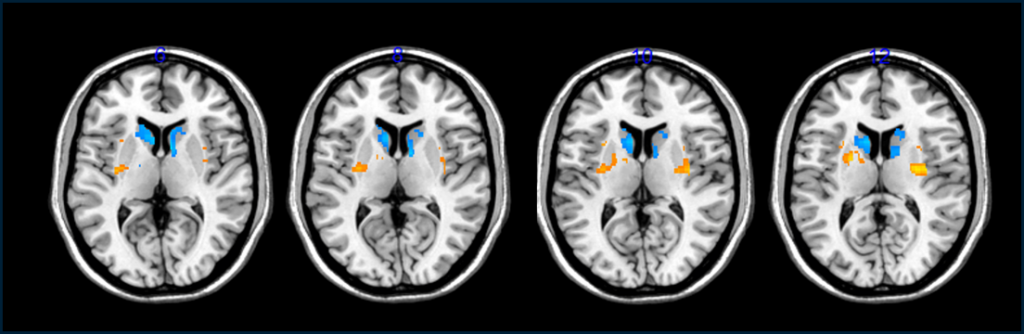
Brain scans of the participants unveiled an uptick in dopamine cells and a reduction in the instances when patients experienced severe symptoms, commonly referred to as “off time.” Remarkably, those who received higher doses of these experimental stem-cell neurons reported fewer episodes of debilitating symptoms.
Although the primary success of this relatively small-scale trial lies in ensuring the procedure’s safety, the ultimate ambition is far-reaching. Researchers aspire to have these lab-grown neurons seamlessly integrate with the host’s brain, functioning as if they were naturally occurring cells.
However, experts like Roger Barker, a Parkinson’s specialist from the University of Cambridge, who was not part of the study, urge caution. He points out that as transplanted cells cannot be directly observed within the brain, confirming their functionality is challenging. To assess their activity, researchers injected a radioactive dopamine precursor, detectable through PET scans during its uptake. While some increased dopamine activity was noted, Barker emphasizes that it’s still too early to ascertain whether these transplanted cells have initiated brain repairs in the patients.
Nevertheless, Barker finds the trial encouraging, highlighting the absence of safety concerns and the potential benefits observed. Parkinson’s management predominantly revolves around symptom alleviation through medication, as there is no cure for this progressive disorder characterized by motor difficulties, tremors, and limb rigidity. Cell therapy, such as the one developed by BlueRock involving lab-grown neurons, promises to revolutionize disease treatment by replacing damaged neurons rather than merely addressing symptoms.
Seth Ettenberg, President and CEO of BlueRock, envisions a future where individuals no longer define themselves by their Parkinson’s diagnosis, stating, “There is a day when we hope that people don’t think of themselves as Parkinson’s patients.”
BlueRock is now advancing into Phase 2 trials, and if successful, this pioneering regenerative technology could inch closer to becoming a mainstream Parkinson’s treatment.