Scientists in the US have made a new type of drug that can regenerate cells and improve paralysis in mice that have spinal injuries. These mice were walking within four weeks of the drug introduction in their bodies.
The research was published in the journal Science on Thursday, and the team of Northwestern University scientists is aiming to get approval from the Food and Drug Administration (FDA) by next year.
“The aim of our research was to develop a translatable therapy that could be brought to the clinic to prevent individuals from becoming paralyzed after major trauma or disease,” Northwestern’s Samuel Stupp, who led the study, told AFP.
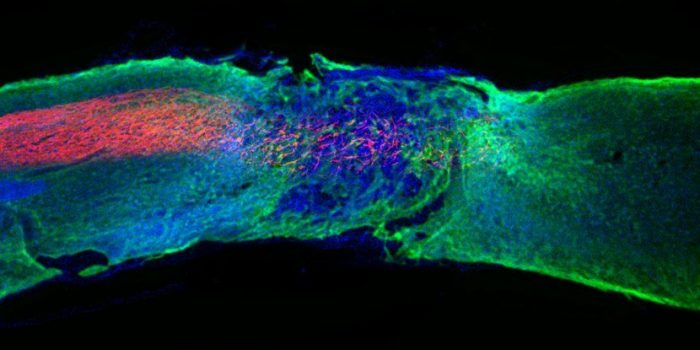
Earlier, stem cells were used to regenerate the cells. This team of researchers has made use of nanofibers to emulate the structure of the extracellular matrix. Each fiber is around 10,000 times thinner than human hair. They are further made up of numerous bioactive molecules called peptides that transmit signals to promote nerve regeneration.
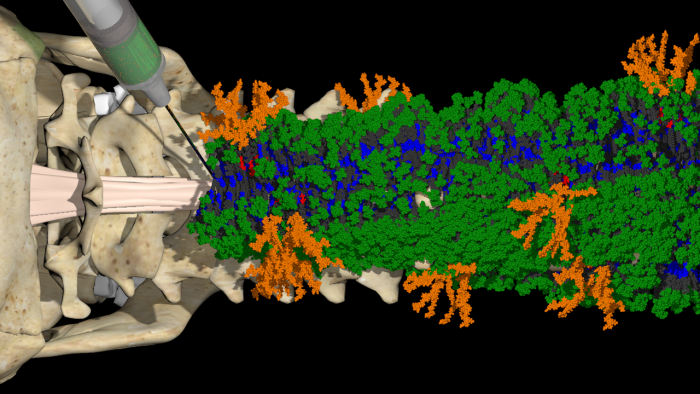
The drug was inserted into the spines of the mice in the state of gel. The spines were incised a day before to make the results more practical. After four weeks, the mice injected with the drug were able to walk again. The rest were not.
It was observed that the axons were regenerated, the scar tissue was diminished, and the axon layer called myelin had formed again with blood vessels recovering themselves as well. A particular mutation in the molecules led to an intensified collective motion and heightened efficacy of the mice.
This is because receptors in neurons are naturally in constant motion, Stupp explained, and increasing the motion of the therapeutic molecules within the nanofibers helps connect them more effectively with their moving targets. It was declared that the mice treated with mutation showed more promising results.
The drug is categorized among the “supramolecular drugs,” because the therapy is an assembly of many molecules rather than a single molecule, said Stupp.
The team wants to move the trials to humans as soon as possible because, according to the scientists, “there is nothing out there to help spinal cord injury patients, and this is a huge human problem,” he said.
It is stated that there are 300,000 people living with a spinal cord injury in the United States alone. Their lifespans or quality of life are not really being improved.
“The challenge will be how the FDA will look at these therapies because they’re completely new,” predicted Stupp.