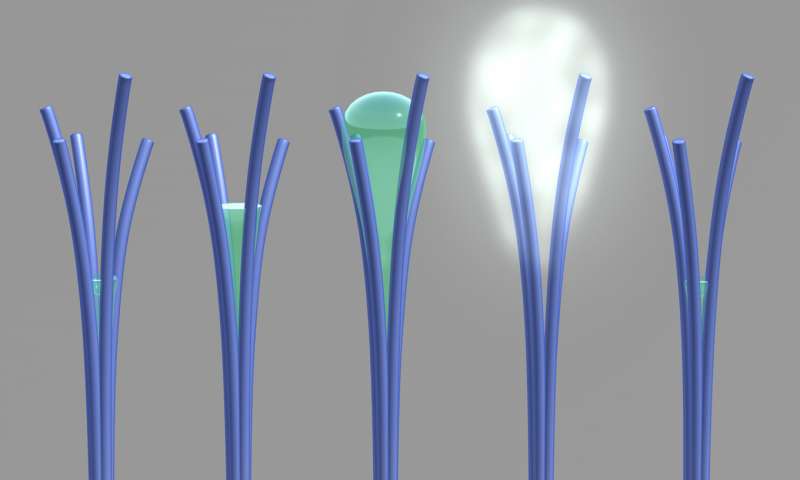
Often in science, the most interesting experiments are the ones that don’t turn out to be as expected. The chemist Satish Nune underwent the same experience when he unexpectedly discovered carbon-rich nanorods while trying to fabricate magnetic nanowires. Intrigued, he decided to analyze them with the vapor analysis equipment, only to find that there was a considerable loss of weight.
When observed under a powerful microscope, Nune and his fellow researchers were amazed to witness the appearance of an unknown liquid between the tiny sticks. The liquid oozes out of the sticks as shown in this video below:
On the release of the fluid, the weight of the nanorods dropped to half their original mass. The research team has now published a paper in the journal “Nature Nanotechnology” which explains the experimental evidence of a phenomenon that had been hypothesized about decades ago. David Lao is a post-doctoral research associate at PNNL, who was included in the design team that made this material.
“Our unusual material behaves a bit like a sponge; it wrings itself out halfway before it’s fully saturated with water.”
Another PNNL engineer named David Heldebrant explained the expectations from this discovery:
“Now that we’ve gotten over the initial shock of this unforeseen behavior, we’re imagining the many ways it could be harnessed to improve the quality of our lives. But before we can put these nanorods to good use, we need to be able to control and perfect their size and shape.”
The Behavior of the Carbon-Rich Nanorods
In real life, the water content in the materials rises as the humidity increases. However, these carbon-rich nanorods expel a large quantity of water when the humidity level rises to 80 percent.
The expelled water is visible as a cloudy, gray mist originating from the intersection of the nanorods. On the further increase in humidity, the weight of the nanorods also rises. Similarly, the lowering of humidity led to an ejection and then reabsorption of water.
The Original Hypothesis
The anomalous behavior of the water vapors intrigued the researchers endlessly. They finally got their hands on a paper published in the Journal of Physical Chemistry B in the year 2012. The paper details a phenomenon where the liquid can spontaneously evaporate as it is confined in a very narrow space. Another study published in the Journal of Chemical Physics in 2013 explain that the liquid can readily condense in the hydrophobic materials to form the vapors. This condensation is spurred by the attractive forces of the two layers of the hydrophobic material. The technical name for this phenomenon is solvent cavitation under solvo-phobic confinement.
This phenomenon was first observed by the scientists who were conducting a study on the crystallized proteins. They noticed that the liquid water was present around the non-hydrophobic areas of the protein while the hydrophobic areas were surrounded by the water vapors.
Thus, the research team from PNNL suggested that the capillary condensation water is responsible for the condensation of water between the nanorods. They also suggested that the adjacent nanorods are pulled together to form a bunch by the surface tension of the water in the rods.
Applications
The discovery of this phenomenon is expected to revolutionize the water purification and separation industry. Thus, the future bids low-energy water harvesting and purification as well as the smart fabric that could absorb the sweat released by the body and release it as vapors.
What other applications can you think of? Let us know in the comments section.