Elon Musk’s neurotechnology company, Neuralink, announced that its experimental brain-chip implant, designed to restore vision, has been granted the U.S. Food and Drug Administration’s (FDA) “breakthrough device” designation.
Neuralink’s experimental device, Blindsight, is designed to help those who have lost their eyes and optic nerve regain their vision. In a post on X (formerly known as Twitter), Musk highlighted the promise of this device, stating that it “will enable even those who have lost both eyes and their optic nerve to see.” However, neither Neuralink nor the FDA has provided further details on when human trials of Blindsight might begin.
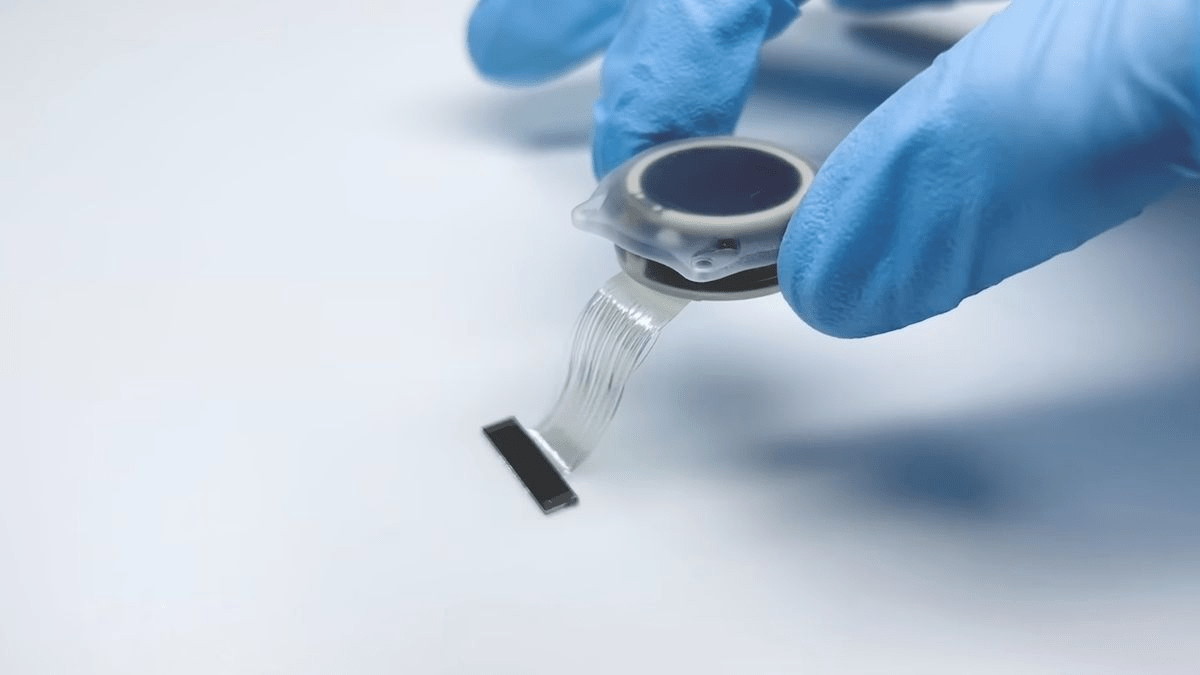
Founded by Musk in 2016, Neuralink is focused on creating brain-chip interfaces that can be implanted in the skull. The company envisions these chips as potential solutions for enabling communication and movement in disabled individuals and restoring lost sensory functions like vision.
In addition to Blindsight, Neuralink is also working on another implant aimed at giving paralyzed patients the ability to operate digital devices using only their thoughts. This technology holds immense promise for individuals with spinal cord injuries, allowing them to interact with computers and phones without physical movement.

Currently, Neuralink is preparing to enroll three patients in a clinical trial to assess this implant’s capabilities. The study is expected to take several years, according to information available in the U.S. government’s clinical trials database.


