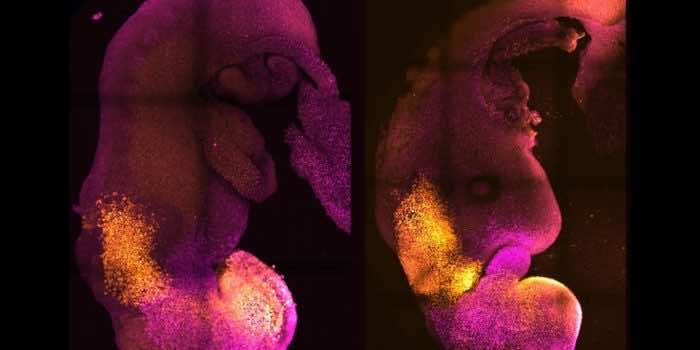
Scientists created ‘synthetic’ mouse embryos that developed a brain, nerve cord, and beating heart tissue without the need for a fertilized egg or uterus for it to grow.
It is like the breakthrough made by another team earlier this month. Together, these developments pave the road to revolutionize the understanding of one of biology’s greatest challenges: how a few cells organize themselves into life.
If this is applied to human embryos, the research could help better understand human fertility, and developmental disorders. This will provide a new way to develop tissues, or organs, for transplantation grown in the lab.

However, implementing this on human embryos would raise important ethical and legal questions.
“The big question we’re addressing in the lab is how do we start our lives?” says Professor Magdalena Zernicka-Goetz from Caltech in Pasadena, California, and the University of Cambridge in the UK.
To create synthetic embryos, or “embryoids”, the scientists took three types of stem cells from a mouse embryo which would normally go on to form all the tissues required in a growing embryo.
They then transferred the cells into an artificial growth medium in a rotating flask of nutrients.
The stem cells spontaneously formed embryos.
Only about one in 100 were successful, but the few that were “are absolutely indistinguishable in many cases from natural embryos” says Prof Zernicka-Goetz.
The embryos only developed for eight-and-a-half days which is almost halfway through the normal gestation period for a mouse.
The team is currently working actively on a human embryo model. However, they are not making similar observations. There are substantial differences between early mouse and early human development.
If it works, having a synthetic human embryo could be a major advance for the study of fertility and common developmental disorders.
“The majority of human pregnancies are lost at the very early stages of our lives,” says Prof Zernicka-Goetz, “and IVF fails in 20 to 70% of cases.”
Supplies of donated human embryos are not many in number and often of poor quality, so a lab-grown “model” embryo could help answer many questions.
The team is proposing synthetic embryoids that emulate only one element of an early human embryo, the heart for example, or the tissue that forms the placenta during implantation. Failure at implantation is a major reason for the failure of IVF pregnancies.

Synthetic human embryos could generate new tissues or organs for “regenerative” medicine. If derived from a patient’s own stem cells, they could be a perfect match for the recipient.
However, developing synthetic human embryos would, in the UK at least, require a change in the current law which does not cover growing embryos from stem cells.
Also, UK law does not allow human embryos to be grown in the laboratory for more than 14 days.
“The result does herald that, in the future, similar experiments will be done with human cells and that, at some point, will yield similar results,” says Prof Alfonso Martinez Arias of Universitat Pompeu Fabra in Barcelona who was not connected with the research.
“This should encourage considerations of the ethics and societal impact of these experiments before they happen,” he added.