Scientists are using the mechanism involved in a natural process of photosynthesis to capture carbon dioxide at 100 times the rate of current technologies. Known as “Artificial Leaf”, these systems could play a vital role in the fight against climate change.
Several different processes involving “artificial leaf systems” have been noticed over the years that use sunlight to turn water into liquid fuels and electricity Similarly, engineers at the University of Illinois Chicago had given a unique design for use in the real world that could work with carbon dioxide from pressurized tanks.
A standard artificial photosynthesis unit was implemented in a solution that was enclosed in a transparent capsule filled with water, and a semi-permeable outer layer. By the absorption of sunlight, water was evaporated through the pores in the outer layer and Carbon dioxide was drawn in to replace it. The unit turned Carbon dioxide into carbon monoxide. Carbon Monoxide could in turn be captured and used to make fuels.

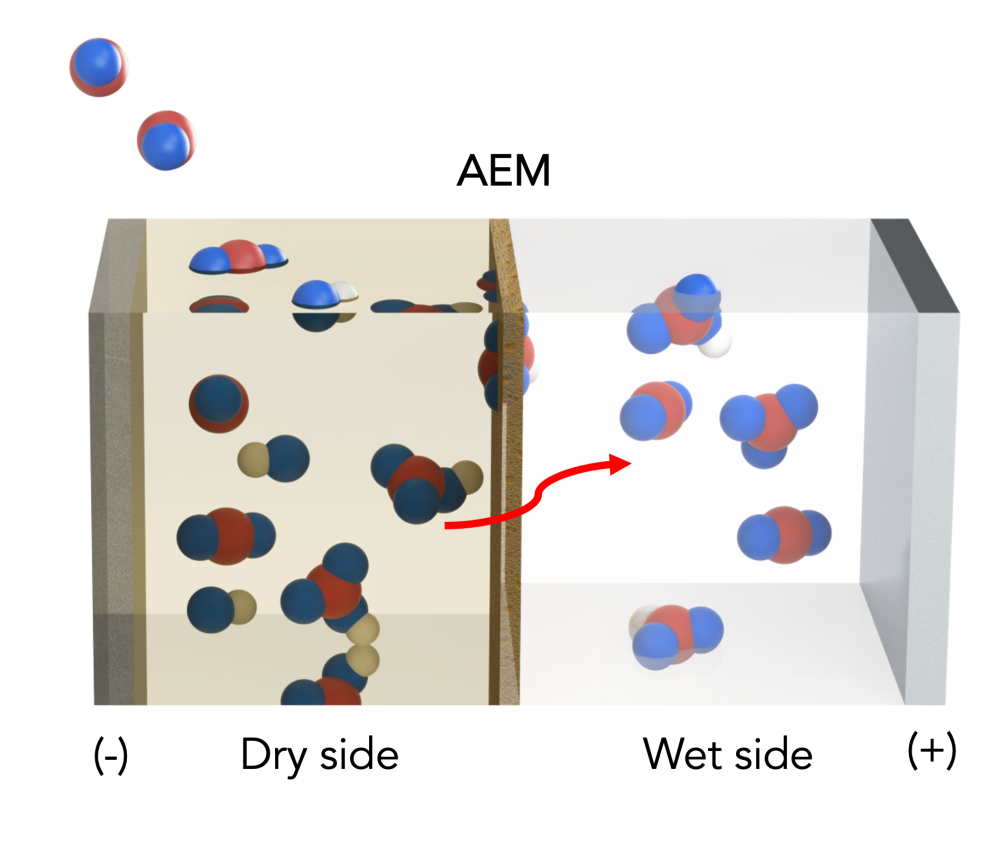
By introducing slight changes, scientists have now taken its performance to new heights. They have installed an electrically charged membrane that acts as a water gradient, with both dry and wet sides. As far as its dry side is concerned, it is made negatively charged and an organic solvent attaches to the captured Carbon dioxide and transforms it into concentrated bicarbonate, which builds upon the membrane.
A positively charged electrode on the wet side then draws the bicarbonate solution across the membrane and into the watery solution. The reaction between the two has resulted in the formation of carbon dioxide. This carbon dioxide can be used to make fuels. Altering the electric charge can accelerate or decelerate the rate of carbon capture, which scientists found at its optimum could capture 3.3 millimoles per hour for every four sq. centimeters of material. The “flux rate” is more than 100 times better than existing systems. As far as the amount of energy is concerned, a negligible amount of it was required to boost the reactions, at 0.4 kilojoules per hour. The team says the system can capture Carbon dioxide at a price of $145 a ton, which is within the Department of Energy’s guidelines that these technologies should cost $200 per ton.
“Our artificial leaf system can be deployed outside the lab, where it has the potential to play a significant role in reducing greenhouse gases in the atmosphere thanks to its high rate of carbon capture, relatively low cost and moderate energy, even when compared to the best lab-based systems,” said Meenesh Singh, assistant professor of chemical engineering in the UIC College of Engineering and corresponding author on the paper.
This whole setup is modular by nature. Multiple units can be stacked on top of one another to build out the devices suited for different settings.“It’s particularly exciting that this real-world application of an electrodialysis-driven artificial leaf had a high flux with a small, modular surface area,” Singh said.


