BiVACOR has achieved a major milestone by implanting its fully mechanical heart into a human for the first time. This pioneering procedure, carried out at the Texas Heart Institute and overseen by the US Food and Drug Administration (FDA), marks a significant step forward in life-support technology for patients waiting for heart transplants.
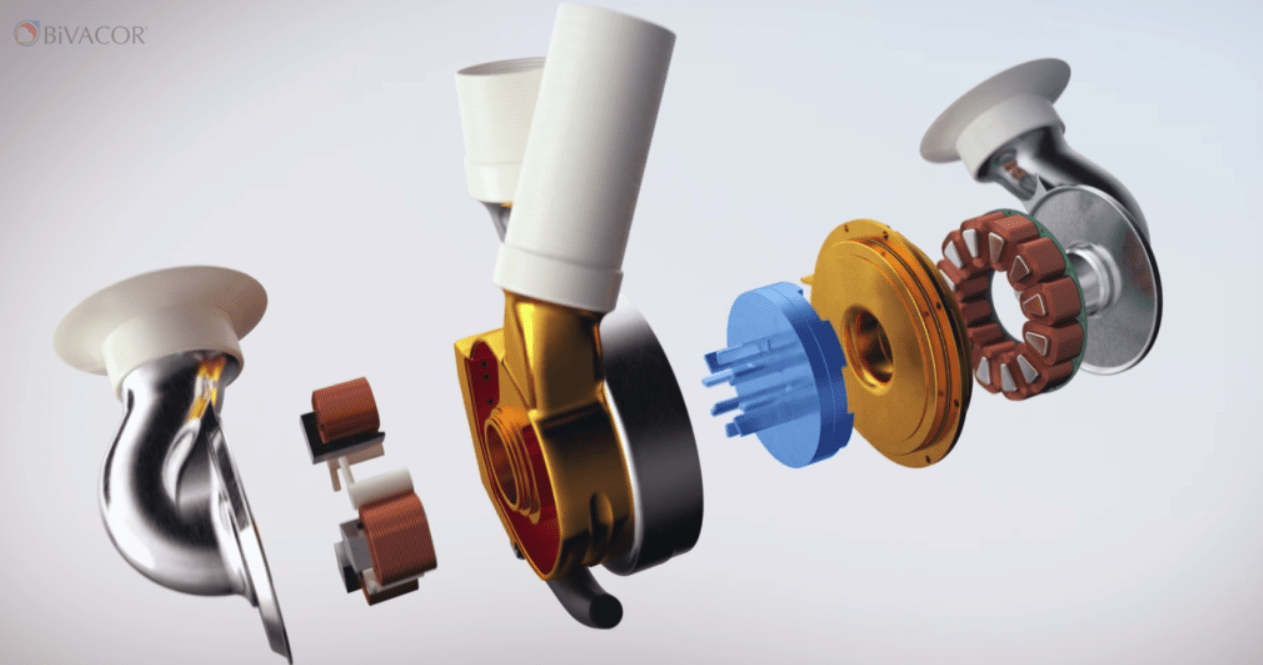
The BiVACOR total artificial heart (TAH) is a titanium-constructed biventricular rotary blood pump. It features a single moving part—a magnetically levitated rotor—that handles the pumping function, replacing both ventricles of a failing heart. This innovative design, which utilizes maglev technology, minimizes friction—a common issue that can degrade mechanical components and affect their durability. Although artificial hearts have been used since the first successful implant in 1969, BiVACOR’s approach with maglev technology represents a novel advancement.
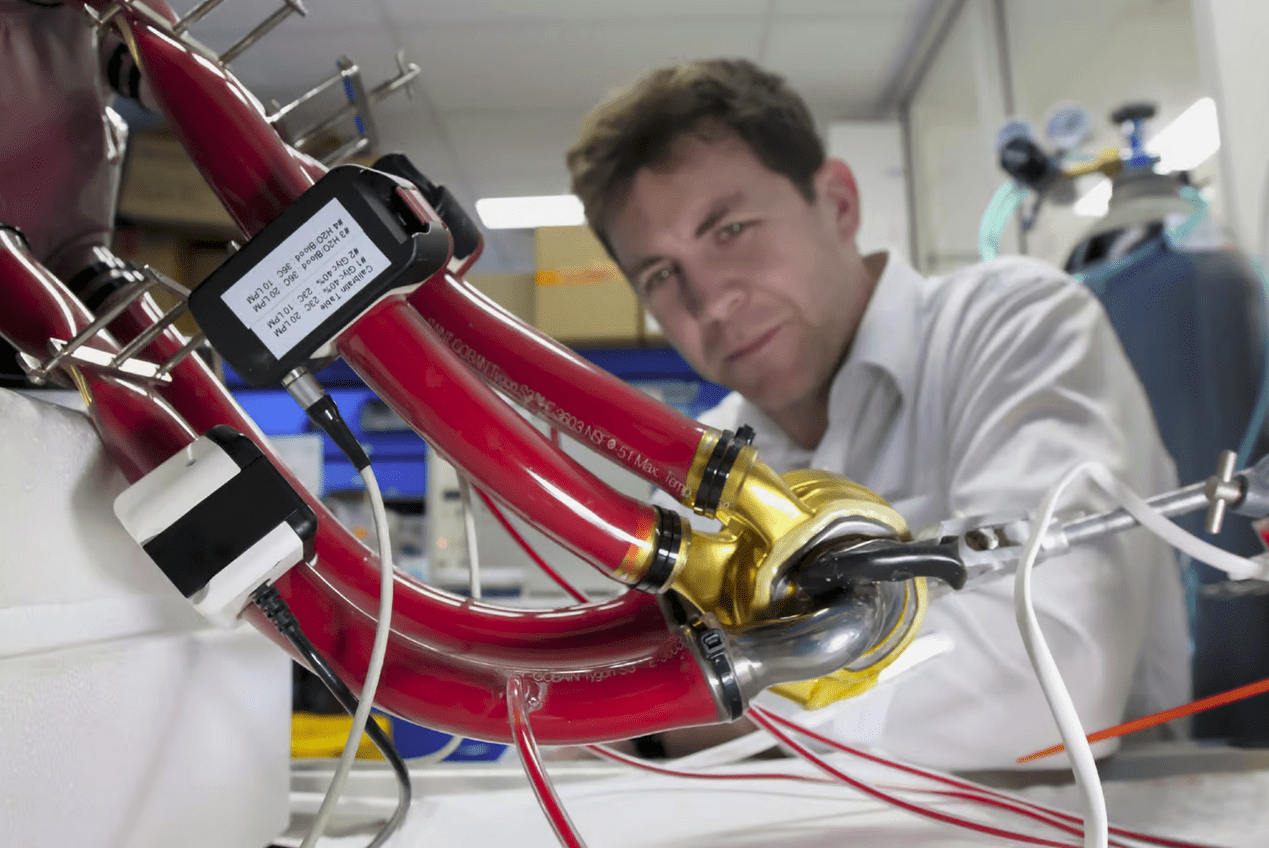
The TAH, about the size of a fist, is powered by a small rechargeable external controller. It can pump blood at a rate of 12 liters per minute, sufficient to support an adult male through exercise. Unlike other artificial hearts that use flexible polymer diaphragms, which can wear out over time, BiVACOR’s device relies solely on its magnetically levitated rotor and has no valves. This design aims to enhance durability, though the titanium heart is intended to serve as a temporary solution while patients wait for a permanent transplant.
Daniel Timms, founder and CTO of BiVACOR, expressed gratitude towards the patient, their family, and the Texas Heart Institute team for their role in this achievement. Timms emphasized that the use of advanced maglev technology brings the company closer to providing a crucial option for individuals with end-stage heart failure who are in need of support before receiving a transplant. The next phase of the clinical trial will involve implanting the TAH in two additional patients for further monitoring.
“This achievement would not have been possible without the courage of our first patient and their family, the dedication of our team, and our expert collaborators at The Texas Heart Institute,” said. Daniel Timms. “Utilizing advanced maglev technology, our TAH brings us one step closer to providing a desperately needed option for people with end-stage heart failure who require support while waiting for a heart transplant. I look forward to continuing the next phase of our clinical trial.”
Heart failure affects at least 26 million people worldwide, and the need for heart transplants has doubled over the past 30 years. Currently, over 3,400 people are waiting for a transplant. If the BiVACOR titanium heart works well in clinical trials, it could significantly increase the chances of survival for many of these patients.


