As the monkeypox outbreak grows, the World Health Organization (WHO) declared the disease a public health emergency, and a Danish biotechnology firm Bavarian Nordic gained an extension of its vaccination approval.
After only applying for the extended label last month, Bavarian Nordic’s vaccine was approved to prevent monkeypox. Bavarian Nordic has already given the vaccine, known in the EU as Imvanex, to several European countries. The vaccine, known as Jynneos in the United States, has already obtained monkeypox approval.

With the World Health Organization calling the outbreak a public health emergency, Bavarian Nordic’s vaccine is in high demand. It was authorized by European regulators to prevent monkeypox far faster than their regular 6- to 9-month review period.
The WHO director-general, Tedros Adhanom Ghebreyesus, said in a statement that he called a WHO emergency committee to assess the monkeypox outbreak a month ago. The committee decided at that meeting that the outbreak “did not represent a public health emergency of international concern.”
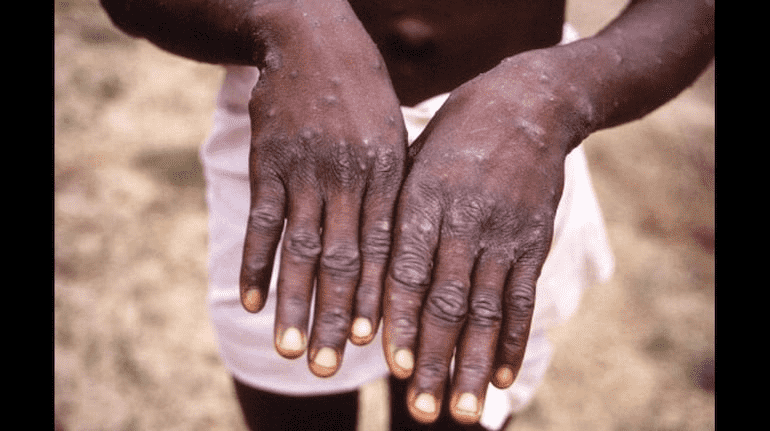
The director-general called a committee meeting to re-assess the situation as the disease spread. A national public health emergency of international concern was declared by Ghebreyesus when the committee could not reach a consensus, focusing in particular on males who have sex with males.
The director-general urged civil society organizations to fight stigma and discrimination in response to the outbreak. “With the tools, we have right now, we can stop transmission and bring this outbreak under control,” Ghebreyesus said.
He advocated for a unified response to prevent the spread of the virus and protect vulnerable groups, as well as efforts to protect affected communities. While the WHO considers the risk of monkeypox to be moderate internationally, it is considered high in Europe.
COVID-19 and polio are the only other ongoing WHO-designated public health emergencies. Since 2007, this has been the seventh public health emergency announced by WHO.


