In a groundbreaking development for regenerative medicine, scientists have harnessed the potential of tiny biological robots crafted from living human cells. These self-assembling entities, known as anthrobots, exhibit remarkable capabilities, showcasing promise in the realms of healing and disease treatment. This achievement builds upon the researchers’ prior success in creating Xenobots from frog embryonic cells in 2020, pushing the boundaries of biological robotics.
The journey towards anthrobots began with the researchers questioning whether the unique capabilities of Xenobots were exclusive to amphibian origins. Seeking diversity, the team embarked on a quest to explore the creation of biobots from human cells. The starting point involved extracting a single adult human cell from the trachea, specifically the windpipe, characterized by cilia—hair-like structures that safeguard the lungs. Manipulating growth conditions in the lab facilitated the replication of these cells into multicellular organoids with outward-facing cilia.
The resultant anthrobots, varying in shape and size between 30 and 500 micrometers, showcased distinct movement patterns based on cilia arrangement—wiggling, traveling straight lines, or moving in circles. Notably, these anthrobots could autonomously maintain their shapes, eliminating the need for external interventions like tweezers or scalpels. Gizem Gumuskaya, the PhD student behind the innovation, highlighted the scalability of the process, emphasizing its potential for mass production as a therapeutic tool.
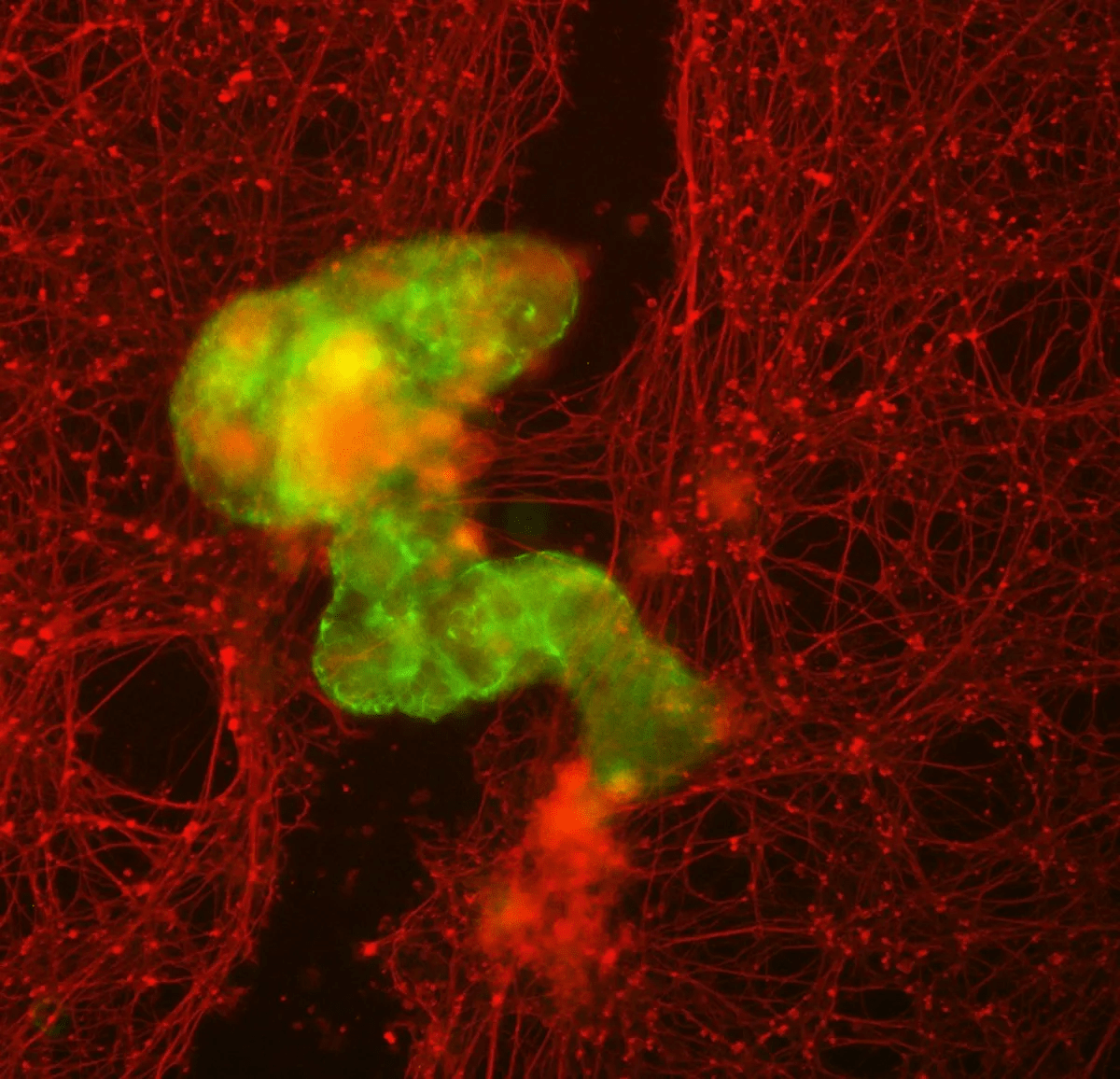
One striking observation was the anthrobots’ impact on damaged neurons in the lab. When introduced to a cluster of neurons, the anthrobots, collectively termed “superbots,” stimulated new cell growth, demonstrating potential healing applications. Despite being composed of tracheal cells, not neurons, the anthrobots displayed unexpected capabilities, opening avenues for neurological diseases, tissue damage, and drug delivery applications.
Gumuskaya underscored the significance of cellular communication and dynamic structure creation, emphasizing the multifunctionality programmed within each cell. Michael Levin, the senior author, expressed fascination at the unexpected ability of normal patient tracheal cells to encourage neuron growth without genetic modification.
The study, published in the journal Advanced Science, signals a paradigm shift in understanding biological body plans and functions, paving the way for innovative advancements in regenerative medicine.