A specific variant of trioxide has been uncovered in the Earth’s atmosphere by researchers from the University of Copenhagen, and it could pose a threat to human life. According to the researchers, these trioxides, which are formed as a result of the reaction of three oxygen atoms with the atmosphere, are more toxic and deadlier than the peroxides, which have only two oxygen atoms. Oxygen is present in a very large quantity on Earth and is also the main resource for sustaining human life. However, as soon as its concentration gets disturbed by the normal setting, it becomes a deadly and poisonous gas for living beings.
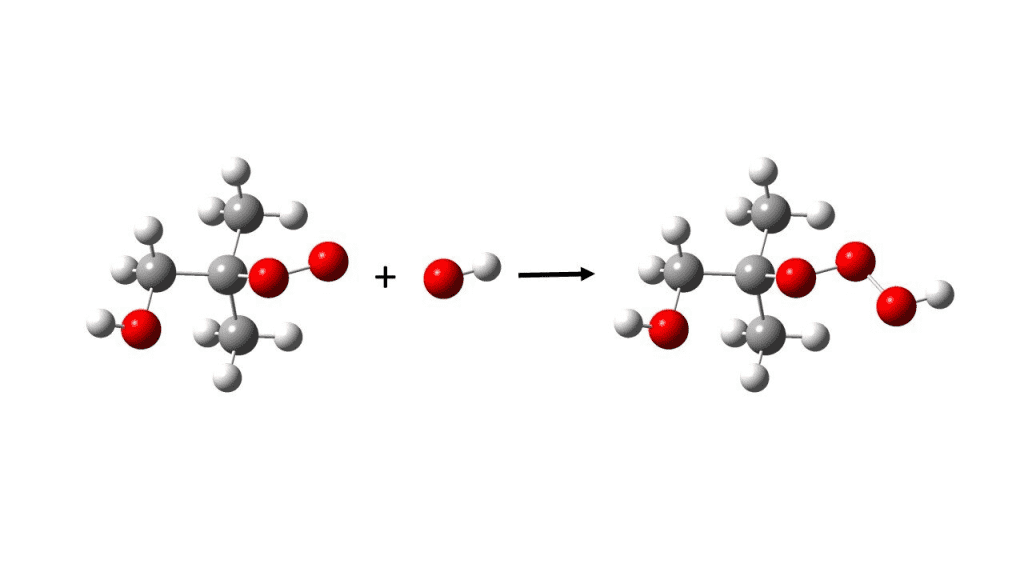
Unlike peroxides, which are verified to be existent in our atmosphere, the presence of trioxide in normal atmospheric conditions in our environment has not yet been proved. But these trioxides come from a class of hydrotrioxides (ROOOH), which encompasses one unpaired electron as well. However, Professor Henrik Grum Kjrgaard, at the University of Copenhagen’s Department of Chemistry, said, “This is what we have now accomplished. The types of compounds we discovered are unique in their structure. And, because they are extremely oxidizing, they most likely bring a host of effects that we have yet to uncover. “
At first, the researchers were unclear about the phenomenon through which these reactive compounds become accessible in our atmosphere. To demonstrate this, they have conducted a lab experiment in which a tube equipped with a continuous jet flow along with precise mass spectrometers and one barometer pressure is enclosed at room temperature, eventually demonstrating that several substances like isoprene and dimethyl sulfide decompose in the atmosphere during their emission, which becomes one of the main reasons for the formation of hydrotrioxide.
The research indicates that out of all the isoprene emitted from different sources in the atmosphere like plants and animals, only one percent of it gets converted into dangerous hydrotrioxide compounds. It has also been extracted from the research that these hydrotrioxides are present in about 10 million per cubic centimeter in our atmosphere for some minutes to hours. This means that their duration of atmospheric existence is short, but is very harmful to the living habitat.
Jing Chen, a Ph.D. student in the Department of Chemistry, stated, “We can now show, through direct observation, that these compounds actually form in the atmosphere, that they are surprisingly stable, and that they are formed from almost all chemical compounds. All speculation must now be put to rest.”
As for the health considerations which are adversely affected by these particles, the researchers said that the hydrotrioxides pass through the tiny particles of aerosols in the atmosphere and get inserted into them. This in turn damages our respiratory system and brings about different heart diseases.
It has further been verified by Professor Henrik Grum Kjrgaard, “They will most likely enter aerosols, where they will form new compounds with new effects. It is easy to imagine that new substances are formed in the aerosols that are harmful if inhaled. But further investigation is required to address these potential health effects.”


