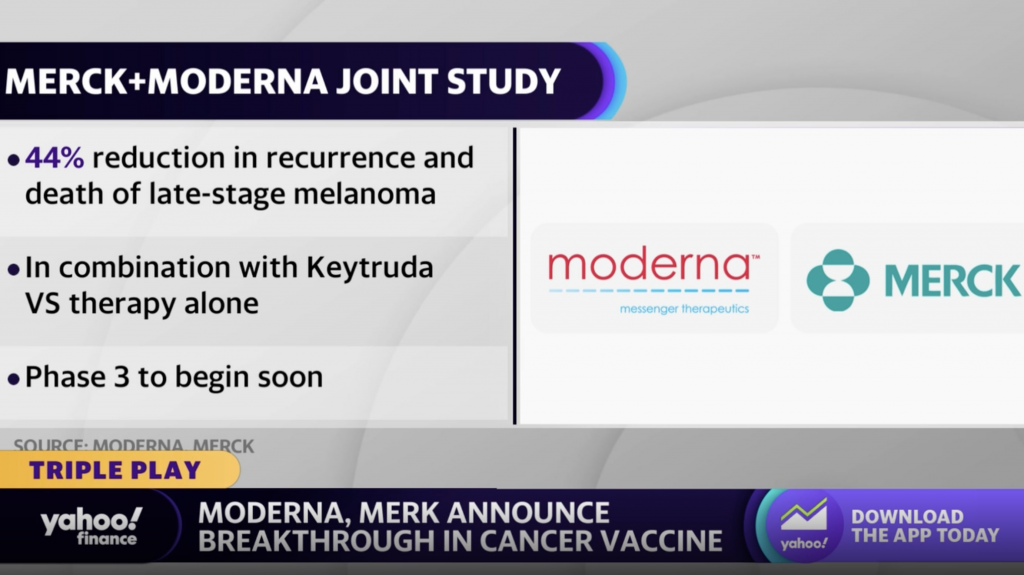
On Tuesday, Moderna and Merck announced the success of an investigational personalized mRNA cancer vaccine, in combination with KEYTRUDA, Merck’s anti-PD-1 therapy, according to a press release.
The combination of these two elements saw the adjuvant treatment of patients with stage III/IV melanoma following complete resection reduce the risk of recurrence or death by 44 percent.
“Today’s results are highly encouraging for the field of cancer treatment. mRNA has been transformative for COVID-19, and now, for the first time ever, we have demonstrated the potential for mRNA to have an impact on outcomes in a randomized clinical trial in melanoma,” said Stéphane Bancel, Moderna’s Chief Executive Officer.
“We will begin additional studies in melanoma and other forms of cancer with the goal of bringing truly individualized cancer treatments to patients. We look forward to publishing the full data set and sharing the results at an upcoming oncology medical conference, as well as with health authorities.”
“These positive findings represent an important milestone in our collaboration with Moderna,” said Dr. Dean Y. Li, president, of Merck Research Laboratories.
“Over the last six years, our teams have worked closely together combining our respective expertise in mRNA and immuno-oncology with a focus on improving outcomes for patients with cancer. We look forward to advancing this program into the next phase of development.”
“The results of this randomized Phase 2b trial are exciting for the field. These data provide the first evidence that we can improve the rates of recurrence-free survival achieved by PD-1 blockade in resected high-risk melanoma. These findings also provide the first randomized evidence that a personalized neoantigen approach may be beneficial in melanoma,” said Jeffrey S. Weber, MD, PhD, principal investigator of the study and Deputy Director of the Perlmutter Cancer Center at NYU Langone. Dr. Weber is a paid consultant for Merck and Moderna.

The new findings were coherent with the results acquired from a Phase 1 clinical trial. The safety profile of KEYTRUDA was consistent with that observed in previously reported studies and even showed a decrease in side effects. Serious treatment-related adverse events were noted in only 14.4 percent of patients who received the combination arm of the mRNA vaccine and KEYTRUDA compared to 10 percent with KEYTRUDA alone.
A Phase 3 study in melanoma patients in 2023 is being prepared for, once approved by regulatory authorities.
Moderna and Merck’s vaccine is designed to stimulate an immune response by generating specific T-cell responses based on the unique mutational signature of a patient’s tumor.
The vaccine has been combined with KEYTRUDA, an immunotherapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells.


