Scientists now suggest that thioacetone is the most stinky chemical substance globally, and this is just the beginning.
Unattended public washrooms, rotting road-kills, and garbage cans are something nobody would want to linger around for long, but they do not even come close to thioacetone on the ‘stinko-meter.’
This pungent-smelling compound is quite unstable and quickly turns to tri-thioacetone above -200C, but on occasions when it does not, it actually poses a mortal threat to those exposed to it for long.
The hazards it poses can be evaluated from the fact that when in 1889, in Freiberg Germany, a factory producing the chemical created city-wide chaos when leakage occurred, and residents described the situation as “an offensive smell spreading rapidly over a great area of the town, causing fainting, vomiting, and a panic evacuation.”
A similar situation occurred in 1967 when a chemical residue of thioketones left uncovered made the workers experience nausea in buildings even at a substantial distance.

In the words of the researchers at the ESO Research station: “Recently, we found ourselves with an odor problem beyond our worst expectations.” They further added that: “During early experiments, a stopper jumped from a bottle of residues, and, although replaced at once, resulted in an immediate complaint of nausea and sickness from colleagues working in a building two hundred yards [180 m] away. Two of our chemists who had done no more than investigating the cracking of minute amounts of tri-thioacetone found themselves the object of hostile stares in a restaurant and suffered the humiliation of having a waitress spray the area around them with deodorant.”
Even Professor Roland Mayer, a pioneer in polymer science working at the Dresden University of Technology, describes the compound as “the smell of this unstable red oil is indeed almost indescribable.”
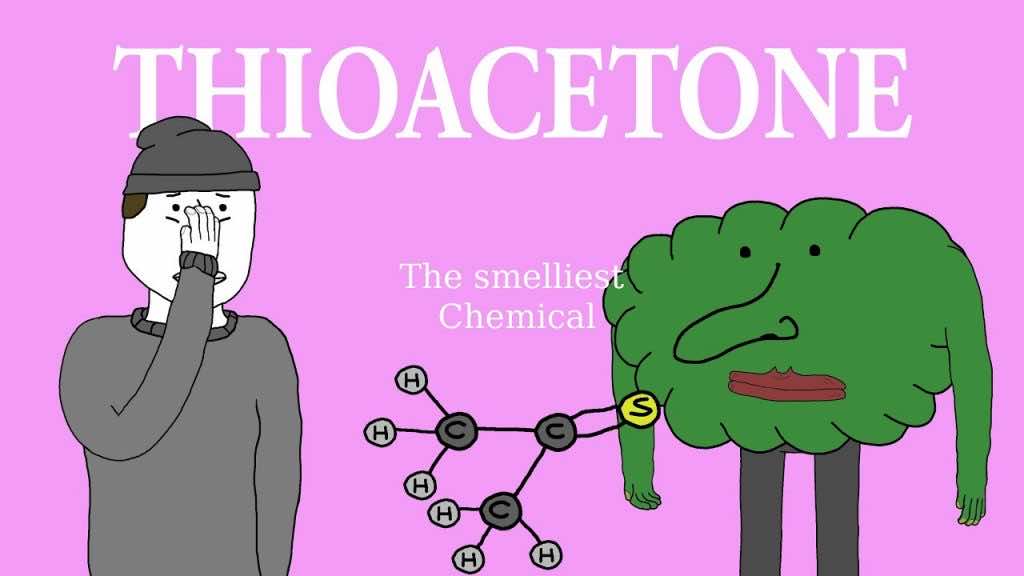
The reason behinds its smelly nature remains a mystery as scientists have failed to cite any precise justification for it. Although the attached sulfur group is the prime suspect, this still does not explain why it smells more than others in its class.
In the words of chemist Derek Lowe, the chemical “Merely stinks. But it does so relentlessly and unbearably”. He described its effects in the following words: “It makes innocent downwind pedestrians stagger, clutch their stomachs, and flee in terror. It reeks to the degree that makes people suspect evil supernatural forces.”

In the views of Benjamin Andrikopoulos, a chemistry student at the University of Melbourne, the aggressive nature of the chemical is somewhat related to evolution. Some people tend to have a stronger sense of smell, and they pass it down the generations and at the same time, historically, sulfur has been notorious for its foul-smelling nature.
Over thousands of years, evolution has made humans smell sulfur even in minimal concentrations, such as parts per million and parts per billion. In the case of thioacetone, the sensing ability of humans is even more profound.
In the words of researchers at the ESO Research station: “The odors defied the expected effects of dilution since workers in the laboratory did not find the odors intolerable … and genuinely denied responsibility since they were working in closed systems”.
Furthermore, they added that: “To convince them otherwise, they were dispersed with other observers around the laboratory, at distances up to a quarter of a mile [0.40 km], and one drop of either acetone gem-dithiol or the mother liquors from crude tri-thioacetone crystallisations were placed on a watch glass in a fume cupboard. The odor was detected downwind in seconds.” And as if all this wasn’t enough, the smell of the pungent compound seems to be a little clingy as it takes quite a while to vanish completely.


