No matter what you collect, I admire your enthusiasm. The courage to seek, the tenaciousness it requires to acquire and to organize your collection takes a lot of effort and dedication. Collecting elements in the periodic table is another story altogether though.
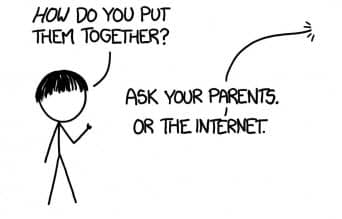
So hypothetically speaking, we did some research and found out here’s what going to happen if you do that. Hence, first of all, how do we acquire the Elements?
30 out of 118 elements in the periodic table are available in pure form and can be purchased from local retail shops, to name a few, Iron, Carbon, Helium, Aluminum and Ammonia. Some can be ordered online and others can be searched by disintegrating things. Samples of 80 elements can be bought and stored or 90 unless you want to be arrested or get injured or sick. Others are either radioactive or transient to collect, and cannot be collected more than a few atoms at a time.
If all the seven rows of periodic table were put together in the same order, here is what will happen;
- Both rows 1 and 2 will not cause any problems.
- The third row will set you ablaze
- The toxic smoke will smother you from row 4.
- All of above, along with a meek dose of radiation will be given off by row 5.
- The row sixth would detonate the building aggressively, releasing radioactive smog and toxic fire and dust.
- You can imagine what happens with row 7. Imagine, Do not try.
1. Row 1
Hydrogen and Helium would float.
- Row 2
Lithium will blemish when in contact with air. Beryllium likewise should not be brought into contact with air, it’s truly toxic. Oxygen and Nitrogen will float around and will slowly diffuse. Fluorine gas would scatter on the floor, being the most corrosive element in the entire periodic table, any substance brought in contact with fluorine combusts instantaneously. Serious damage to your body will be observed if you breathe even a trace of hydrofluoric acid, which is formed when fluorine comes in contact with moisture. Even some gas masks are not a good enough protection in this case.
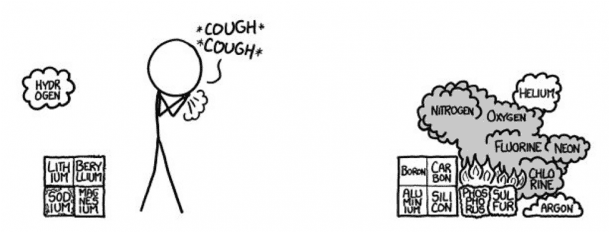
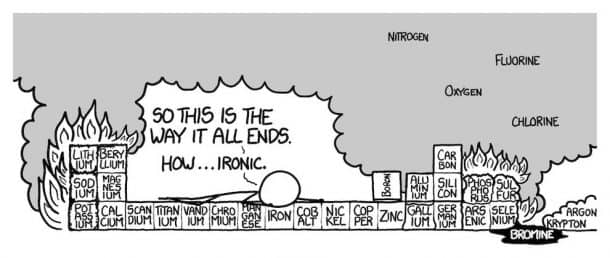
- Row 3
Pure Phosphorous can be available in many forms. Since white Phosphorus combusts when it comes in contact with air, we assume red phosphorus, since it’s safe to handle. Sulfur would smell bad. Since Sulfur is right next to the Fluorine, chances are it would catch fire.
Argon would scatter on the floor because it is denser than air, however, the Sulfur hexafluoride being formed is definitely something you would be worried about. Because, if this experiment is being conducted indoors, there is a good chance that you might choke on the poisonous smoke and even burn down the building.
- Row 4
The entire row is responsible for the revolting odor. Arsenic is poisonous to all life forms and that may sound terrifying, however, traces of natural arsenic are found in all our foods and even water. Let’s roll back to the terrifying part since arsenic in nature and as an element is not the same thing. The flaming Phosphorous could burn arsenic, which can in turn release arsenic trioxide. Two words, Extremely Toxic. Selenium and Bromine are super reactive, although burning Selenium ‘can make Sulfur smell like Chanel’ according to Organic Chemist Derek Lowe.
Say that Aluminum hasn’t caught on fire, The melting Gallium under Aluminum will cause it to become soft as soaked paper. At this point, The burning Sulfur has spilled into the Bromine. Bromine and mercury are only two liquid state Elements at room temperature in the periodic table. There is no safety measure at this point with so many elements ablaze and burning and melting into each other.
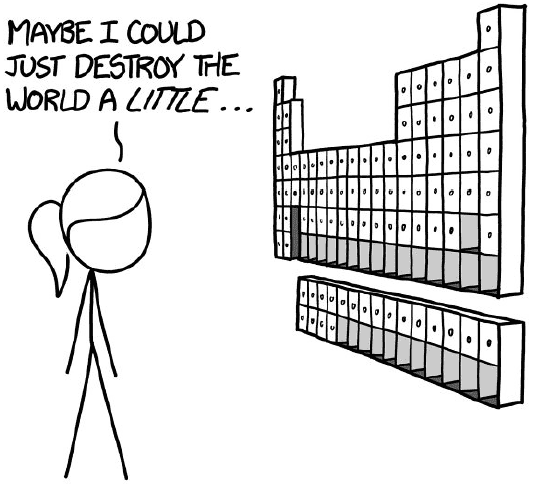
- Row 5
Technetium-99 is the first radioactive elements we study, with no stable isotopes. The amounts anywhere less than 1 liter may not kill you, however, if you breathed in its dust, it will certainly do the job. The row exhibits much similar behavior to row 4.
- Row 6
You can practice all the safety in the world and it will not be enough not to kill you. Talking about radioactive elements in Row 6, let’s dwell on Astatine. We may not have a physical appearance of Astatine for you, because as Lowe would say, ‘that stuff just doesn’t want to exist.’ Even a large block would vaporize by its own heat, quickly. The heat produced would be enough to give 3rd-degree burns to the nearest person, damaging the building severely, leaving a cloud of hot smoke towards the sky, radiating heat. If the explosion was any bigger, you would be roasted along with acute radiation poisoning. Similar is the behavior of the neighboring elements, that’s why they were ‘theoretically’ placed together.
- Row 7
If you thought that Row 6 was bad, you’ve got a whole new thing coming for you. This is the back bench of class, this is where all the riots and revolutions are born. Some of the elements here are not even given a proper, official name and are nicknamed like ‘unununium’. Why would you, when it takes a particle accelerator to create it, and then it decides to exist for only a few minutes. These elements are collectively called Transuranic Elements, and they decay radioactively.
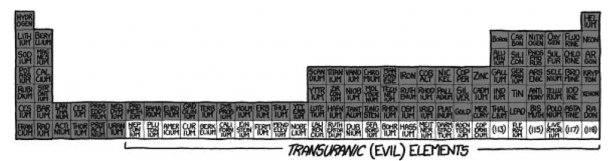
If you were to take any higher numbered element, it would decay in seconds leaving humungous amounts of energy. We are not talking about a nuclear explosion, it won’t be a chain reaction, instead it will happen all at once. The result would be you, along with your bricks and your periodic table turned to Plasma. If you imagined a mushroom cloud, you’d be right. It would resemble a medium-sized nuclear explosion.
As much as I am a fan of collecting things, collecting elements may not be as fun as it may sound.
Credits: Randall Munroe



You know ammonia isn’t an element, right?
This would be a great educational show.
Good post now do one for columns… the gases would be interesting in a stale wind environment.