FDA has recently approved a treatment which can stop a migraine before it even starts. Migraines for many comes in the form of a throbbing headache with physical pain, light sensitivity and nausea are very common. One in seven people often experiences regular migraines which can last for an hour to many days. The treatment of the condition was not very widely available despite it being the most common condition among several people. There is a category of drugs called triptans which can stop a migraine in people mostly but even then there were very few treatments which could successfully prevent a migraine.

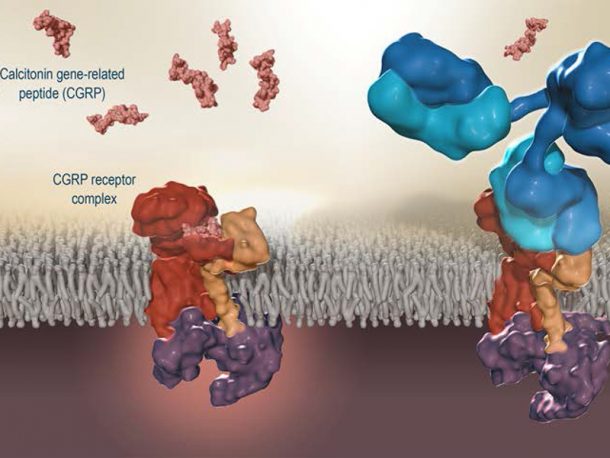
The newly approved drug from FDA is called Aimovig. It was developed in collaboration with Amgen and Novartis. The patients receive monthly injection which blocks the receptor that is attached to a neurotransmitter which has been identified by researchers as playing a key role in migraines. The drug is now available after an extensive research. In the 1980s, the researchers who were working to investigate migraines found that neurotransmitter which was known as the calcitonin gene-related peptide (CGRP), regulate the nerve communication and helps to control the blood vessel dilation. The people who had a migraine had more amount of CGRP molecules in them. It was also discovered that injecting CGRP into people who get migraines, will trigger an attack. People without any history of migraines can also get the same injection without any headache response.
The initial method of creating drugs which blocked CGRP were useless since the molecules were too toxic. Aimovig was developed when scientists were trying to use a new approach of using monoclonal antibodies. These cloned cells attach to receptors on the CGRP cell’s surface and made them useless to the nerve cells. The good thing is that it is very targetted and has very few side effects. The effects are not going to help all the patients. A recent study of the drug showed that most patients experienced only 50% reduction in the migraine episodes. Other patients said that their pain was eliminated completely. For the drug to work on the patient, regularity and consistency are required.
It is required to be taken each month. Drug trials test the effects of the drug on a patient for as long as the trials last so the new users to the basically the guinea pig in the lab. The drug will be available this week. A single dose of the injection costs $575 a year. The drug companies have also announced an assistance program to reduce the cost of the drug to as little as $5 a month.


