Researchers at Columbia Engineering have developed a new type of battery designed to revolutionize the way renewable energy is stored.
This innovative technology, known as K-Na/S batteries, uses abundant materials such as potassium, sodium, and sulfur to create a low-cost, high-energy solution for long-duration energy storage. They achieve nearly maximum capacity at 75°C, delivering 1,655 mAh per gram of sulfur, and with higher sulfur concentrations (up to 4M), the batteries retain 71% of their capacity after 1,000 cycles at a discharge rate of 2mA/cm².
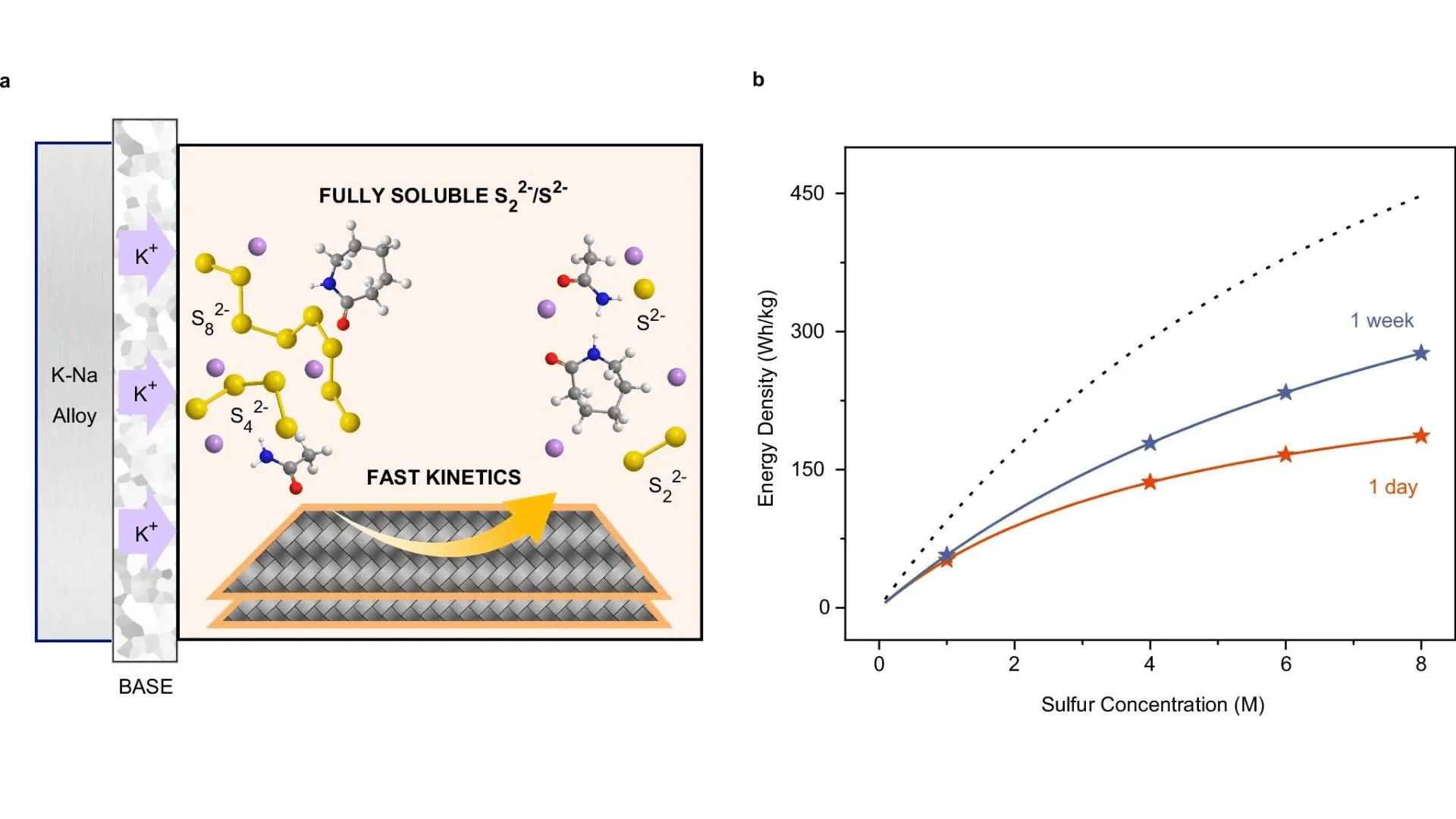
According to the researchers, the batteries offer energy densities of 150–250 Wh per kilogram, making them an ideal choice for long-term energy storage systems. “It’s important that we be able to extend the length of time these batteries can operate, and that we can manufacture them easily and cheaply,” said Yuan Yang, the team leader and associate professor of applied physics and mathematics at Columbia Engineering.
Renewable energy sources like wind and solar are vital for a sustainable future but suffer from a key limitation—they don’t always generate electricity when it’s needed. The challenge lies in efficiently storing this energy to ensure a constant power supply. Columbia’s K-Na/S batteries aim to solve this problem by offering a low-cost, high-energy storage solution using materials that are abundant on Earth.
The team addressed two key issues faced by K-Na/S batteries: low capacity caused by the formation of inactive solid compounds like K2S2 and K2S, and the need for high operating temperatures above 250°C, which complicates the battery’s thermal management and increased costs. Previous attempts to improve battery capacity were hindered by solid precipitates that blocked the diffusion process.

To overcome these obstacles, Professor Yang’s team developed a new amide-based electrolyte. This electrolyte boosts the solubility of K2S2 and K2S, enhancing ionic movement and reaction rates, which significantly improves battery performance. As a result, the K-Na/S batteries operate at lower temperatures (50–100°C) and achieve a higher voltage of approximately 2.1V compared to traditional sodium and potassium sulfur batteries.
“Our approach achieves nearly theoretical discharge capacities and extended cycle life. This is very exciting in the field of intermediate-temperature K/S batteries,” said Zhenghao Yang, co-first author of the study and a PhD student under Professor Yang.
The new electrolyte mixture, which includes acetamide and ?-caprolactam, allows for the dissolution of K2S up to 1.43M at 75°C. This development enables the battery to reach almost full discharge capacity, improving both energy and power density. The researchers’ findings demonstrated that the battery retains 71% of its capacity even after 1,000 cycles, and thanks to the use of widely available materials, it is a cost-effective solution for energy storage.
Although the team is currently focusing on small, coin-sized batteries, they plan to scale up the technology in the future, to store large amounts of renewable energy. “If successful, these batteries could provide a steady and reliable power supply from renewable sources, even during periods of low wind or sunlight,” explained the researchers.
For now, the team’s primary focus is on optimizing the electrolyte composition to further enhance battery performance. With ongoing improvements, Columbia’s K-Na/S battery technology holds great potential to make renewable energy more reliable and accessible for future energy systems.


