Diabetes is one of the most prevalent health problem with 415 million adults suffering from it around the world. The market for diabetes devices has matured to a multi-billion dollar industry through the advancement of technology. The biggest problem with diabetes and its treatment is that it requires needle pricking all the time, whether to monitor blood sugar or to inject insulin. Things have improved slightly after the introduction of insulin patches, but it is still not a perfect solution. The ideal way would be to trigger the body to produce insulin as and when required, just like the natural system works and a team of scientists at East China Normal University has achieved that.
The research led by Haifeng Ye was published in Science Translational Medicine. Inspired by smart home systems, it combines two emerging medicine fields cell-based therapy and optogenetics with telecommunications technology. Optogenetics is a technique that utilizes light for regulating cellular activity. The idea has been applied to restore heart rhythms, reverse blindness, and to activate predatory instincts in mice.
The team customized some cells, enabling them to create insulin when illuminated with light waves from the far-red region. The insulin making process can be triggered in cells using a smartphone to light up far-red LEDs (FRLs). The researchers embedded the lights and the enhanced cells in a biocompatible sheath to implant under the skins of diabetic mice.

The entire system is made up of the genetically engineered cells, an LED control system with an electromagnetic circuit, a smartphone application to control light, and a blood glucose meter that communicates glycemic values to the app via Bluetooth. The glucose meter conducts periodic testing of glucose levels at all time. The app then analyzes these results to determine the required amount of insulin and orders the LED control box to enable the LEDs to illuminate the cells which then begin to produce insulin. The mice were exposed to four hours of light every day which maintained a sustainable level of insulin production continuously for 15 days. Light exposure of just two hours resulted in blood sugar levels of a nondiabetic mouse, with no hypoglycemic side effects.

The research is a continuation of the work Ye began as a Ph.D. student at ETH Zurich where he had used blue light to control glucose levels in mice. Continuous exposure to blue light was found to be toxic to mammals, so the team shifted to FRL instead. The far-red light is commonly used in physiotherapy infrared lamps. This research, in particular, is focused on the treatment of diabetes, but the same idea can be applied to other metabolic diseases.
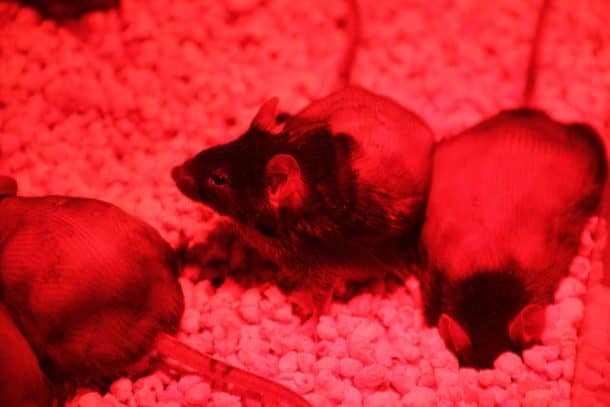
The team has been able to deliver a successful proof of concept, but there is still a lot of work to be done for the system to be applied to humans. The system still requires drawing blood manually and close proximity to the electromagnetic circuit. This limits mobility while exposing them to electromagnetic radiation. Before the system makes it way out of the lab, validation will be required for patients who are undergoing both the traditional treatment and the optogenetic system.
Researchers suggest that the glucometer could be replaced with a continuous glucose monitor implanted in the body. Using clinically approved batteries could allow users more movement by eliminating the electromagnetic coil. The problem remains with the testing of patient cells, and the team is formulating plans to conduct these tests in hospitals.
Source: AAAS
Images: Shanghai Key Laboratory of Regulatory Biology


